🌦 Climate Change
Greenhouse Effect, Global Warming, Ozone Depletion, Eutrophication
- Climate change is any significant long-term change in the weather of a region (or the whole Earth) over a significant period of time.
- Climate change is about abnormal variations to the climate and the effects of these variations on other parts of the Earth.
Greenhouse Effect
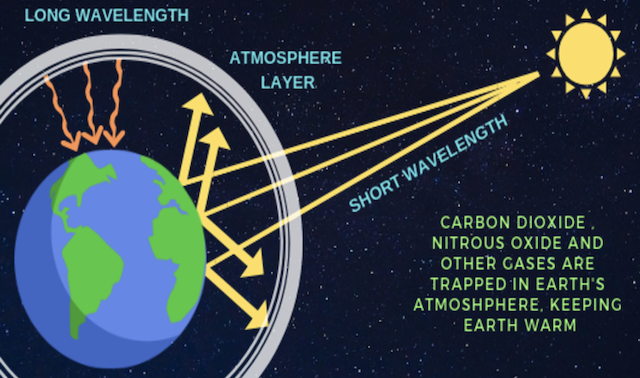
- The greenhouse effect is a process that occurs when gases in Earth’s atmosphere trap the Sun’s heat.
- This process makes Earth much warmer than it would be without an atmosphere.
- The greenhouse effect is one of the things that makes Earth a comfortable place to live.
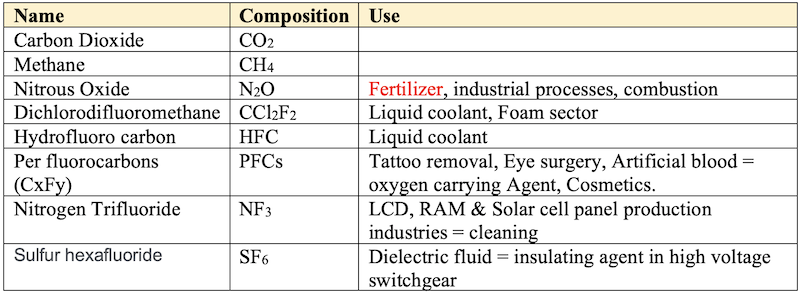
Green House Gasses (GHGs)
Global Warming
“Global warming is the extraordinary increase of Earth’s surface temperature due to the increase of greenhouse gases concentration on the atmosphere.”
- Greenhouse gases are the heat-trapping gases in the atmosphere (carbon dioxide, methane, nitrous oxide, and CFC). They are the fundamental parts of the greenhouse effect, the role played by the atmosphere to continually warm the earth, trapping some portions of heat that came from the solar energy (sun radiation) from reflecting back to space - just like the work of a greenhouse.
Causes of Global Warming
- Rapid use of fossil fuel,
- Rapid use of fossil fuel will emit large amount of greenhouse gases, especially carbon dioxide,
- Deforestation/clearing of lands.
Signs of Global Warming
- The receding of ice formations on Earth (snows at mountain-tops, glaciers, and Antarctic and Arctic ice).
- The increase of shrubbery in Arctic.
- Thinner clouds over the sky that decrease the ability to reflect heat from the sun.
- The discovery of the decrease of Earth’s albedo (the amount of sunlight reflection by the Earth surface to the Moon) by 2.5 per cent, which means the Earth has loosen some levels of capability to reflect sunlight to the Moon.
- Change in wind directions.
Impacts
- Stormy weather (more chances for hurricanes, floods, cyclones, and storms to happen).
- Increased severity for drought, hunger and spread of diseases, especially in poor countries.
- Declines of amphibians, caused by altered precipitation patterns resulted in lower levels of pond and lake waters, where amphibians survive.
- Damages to coral reefs
- Marine diseases
- Rising ocean temperature
- Ecosystem degradation
- Declining of biodiversity
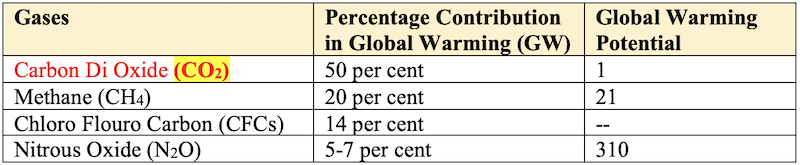
Percentage Contribution of Different Gases in Global Warming
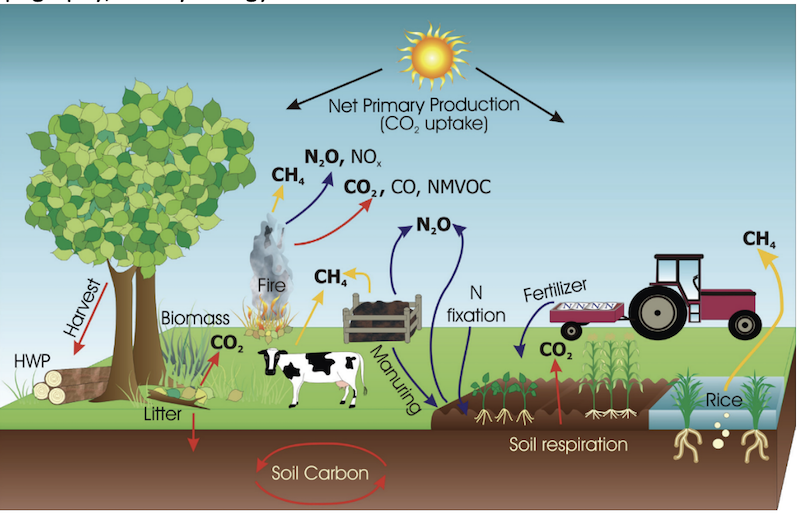
- Farms emitted 6 billion tonnes of GHGs in 2011, or about
13 percentof total global emissions. That makes the agricultural sector the world’s second-largest emitter, after the energy sector (which includes emissions from power generation and transport). - Most farm-related emissions come in the form of methane (CH4) and nitrous oxide (N2O).
- Cattle belching (CH4) and the addition of natural or synthetic fertilizers and wastes to soils (N2O) represent the largest sources, making up 65 percent of agricultural emissions globally.
- Smaller sources include manure management, rice cultivation, field burning of crop residues, and fuel use on farms. At the farm level, the relative size of different sources will vary widely depending on the type of products grown, farming practices employed, and natural factors such as weather, topography, and hydrology.
Solutions
- On the demand side, crop management practices—such as improved fertilizer management and conservation tillage—offer the greatest reduction potential at relatively low costs. Better managing grazing lands—such as by rotational grazing and altering forage composition and restoring degraded lands and cultivated organic soils into productivity are also important.
- On the supply-side, shifting away from meat and particularly beef consumption offers the most potential for reducing emissions. Also, recent WRI research shows that about 24 percent of all calories currently produced for human consumption are lost or wasted in the food supply chain. Consequently, reducing food losses and wastes can also play an important role.
Ozone Depletion
- Ozone is the triplet form of atomic oxygen (O3) in the stratosphere. It lies about 14 miles above earth surface. It acts as a protectant by absorbing the dangerous UV radiations. In stratosphere, contribution of combination and recombination process of oxygen keeps on taking place. Thus stratospheric ozone is constantly maintained.
UV + O3 ↔ O + O2
- Ozone depletion refers the steady declining of ozone concentration in the stratosphere due to it’s in the faster destruction by the different ozone depleting substances environment.
Ozone depleting substances
- Chlorofluoro Carbons (CFCs): These substances contain chlorine or fluorine. These elements are capable of breaking down ozone into oxygen.
Cl + O3 → ClO + O2 O + ClO → O2 + Cl
- This free halogen again reacts with ozone and break down it.
- One single chlorine ion is capable to break down million ozone molecules.
Ozone hole
- Ozone hole is an atmospheric phenomenon whereby the
stratosphereregion contains no or less ozone in the particular region due to extensive depletion of ozone molecules. - It was first discovered by Joe Forman over the Antarctica.
- Impact of ozone depletion
- Area falling under ozone hole is unprotected from UV radiations.
- UV radiations cause damage to health such as skin cancers, cataracts, decreased immunity, etc.
- UV radiation kills the plankton which impact marine ecosystem negatively.
- Many plants are unable to tolerate this radiation.
- O3 depletion also result greenhouse effect in the lower atmosphere.
Acid Rain
- Acid rain is the kind of precipitation that contains larger amounts of acid than normal.
- Rainwater is usually slightly acidic, with pH level between 5 and 6. Water that evaporates from earth is neutral (pH 7) and it becomes weak acid when mixed with carbon dioxide in the atmosphere.
- This is caused by the presence of air pollutants, like sulfur dioxide (SO2 - 70%) and nitrogen oxides (NO2). They produce acids if combined with water.
- Acid rain is considered as the wet deposits of air pollutants, where it’s combined with moisture before falling into the ground.
- Acid rain can occur naturally, from the volcanic eruptions. However we are also causing this, from the emission of vehicles and of industrial plants that include the burning of fossil fuels. If we continue to increase rate of air pollution, we are increasing the risk of acid rain to happen.
Consequences of Acid Rain
- A major environmental impact of acid rain is the lowering of pH in water bodies and soil.
- It destroys the aquatic fauna and flora.
- Phytoplankton populations are reduced.
- Certain forest tree species declines because of soil acidification.
- Acid rain causes corrosion of metals and the deterioration of painted surfaces, concrete, limestone, and marble in buildings and monuments.
- It may cause some skin diseases to human being.
Eutrophication
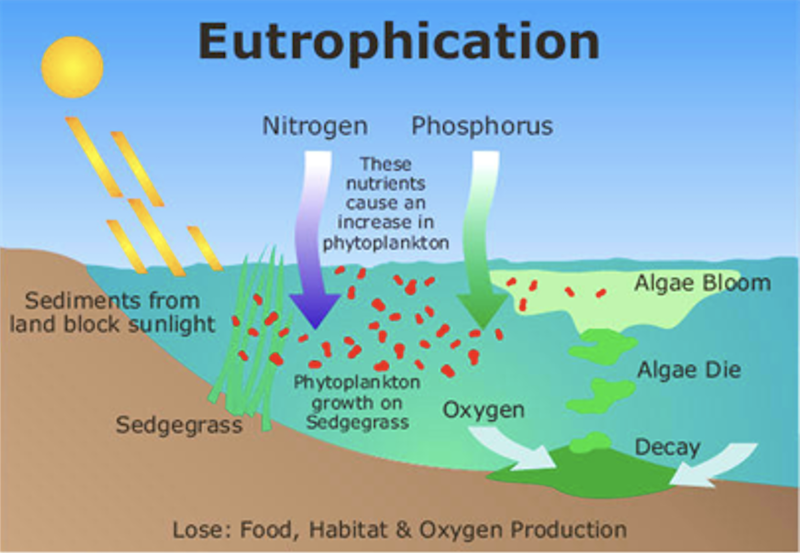
- Eutrophication refers the process of enrichment of water bodies with nutrients through various mechanisms.
- This is due to addition of domestic waste, run-off from agricultural fields (phosphates, nitrates), etc. Thus the water bodies becomes highly productive or eutorphic. This phenomenon is called as eutrophication.
Consequence of eutrophication
- Initial luxuriant growth of plants and phytoplanktons occurs in waterbody due to enrichment of nutrients.
- This encourages further growth of zooplanktons and fishes. Heavy load of organism decreases the oxygen rapidly.
- With progress of time, all living organism will die due to starvation of oxygen.
- Water become black
Measures for eutrophication
- Wastewater must be treated before its discharge into lake or river.
- Stimulation of bacterial multiplication in order to reduce nutrients load.
- Removal of algal blooms upon their death decomposition.
- Removal of dissolved nutrients from water by physical or chemical methods.
- Climate change is any significant long-term change in the weather of a region (or the whole Earth) over a significant period of time.
- Climate change is about abnormal variations to the climate and the effects of these variations on other parts of the Earth.
Greenhouse Effect

- The greenhouse effect is a process that occurs when gases in Earth’s atmosphere trap the Sun’s heat.
- This process makes Earth much warmer than it would be without an atmosphere.
- The greenhouse effect is one of the things that makes Earth a comfortable place to live.
Green House Gasses (GHGs)

Global Warming
“Global warming is the extraordinary increase of Earth’s surface temperature due to the increase of greenhouse gases concentration on the atmosphere.”
- Greenhouse gases are …
Become Successful With AgriDots
Learn the essential skills for getting a seat in the Exam with
🦄 You are a pro member!
Only use this page if purchasing a gift or enterprise account
Plan
Rs
- Unlimited access to PRO courses
- Quizzes with hand-picked meme prizes
- Invite to private Discord chat
- Free Sticker emailed
Lifetime
Rs
1,499
once
- All PRO-tier benefits
- Single payment, lifetime access
- 4,200 bonus xp points
- Next Level
T-shirt shipped worldwide

Yo! You just found a 20% discount using 👉 EASTEREGG

High-quality fitted cotton shirt produced by Next Level Apparel