👨👨👦👦 Classification
Fertilizers, Indian Fertilizer Scene, Classification of fertilizer
Fertilizers
- Liebig in Germany and Lawes in England developed independently the idea of treating phosphate with H2SO4 and producing a water-soluble phosphate by about 1840. It is known as superphosphate or simply super.
- Lawes set up a factory at Deptford in 1843 for the production of superphosphate. Possibly this was the first factory to be set up for the production of artificial fertizers on a commercial scale.
- Fertilizers are the organic or inorganic materials of natural or synthetic origin which are added to the soil to supply certain elements essential to the growth of plants.
- Fertilizers are generally inorganic in origin and they supply one/more essential plant nutrients in large proportions.
- The term ‘Fertilizer’ is now commonly restricted to commercial products.
- Amendments are the substances other than manures and fertilizers which are added to soils for the improvement of their condition.
- Amendments are also termed as ameliorants, improvers or soil conditioners e.g. gypsum and lime though they supply nutrients but the main objective of applying them is for correcting the soil condition.
- Reverse Fertilization FCI AGM 2021: Removal of soil nutrients.
- The relationship b/w water & fertilizer as production factors of crops is synergetic.
- The overall foodgrain production to fertilizer applied ratio is about 10 : 1.
- First Agricultural Chemist of ICAR - J W Leather
Indian Fertilizer Scene
- India is the Third largest producer and consumer of fertilizers in the world.
- At present, there are 59 large size fertilizer plants in the country manufacturing range of fertilizers.
- The current installed capacity is 12.1 m tonnes per annum (TPA).
- This fertilizer sector is highly subsidized. It relies heavily on imports to meet domestic demand.
- The massive subsidisation of fertiliser is also creating several distortions, one in terms of huge cost to the exchequer and other is imbalanced used of fertilisers. The biased use of urea fertilizer due to large amount of subsidy has inefficiency in the optimal usage ratio of NPK fertilisers. This imbalance has also reduced the crop response ratio.
- A systematic approach to tackle this situation can be found by bringing urea under the nutrient based subsidy and introducing direct cash transfer on per hectare basis urea and complex fertilsers.
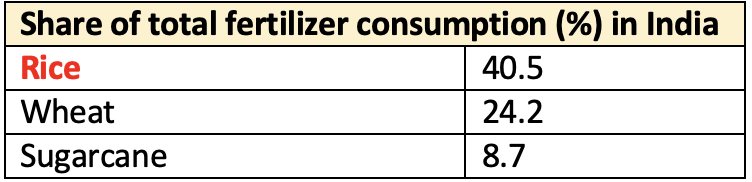
- The major grades of fertilizers are Nitrogenous (N), Phosphatic (P) and Potassic (K).
- Average consumption of fertilizers in India
165.8 kilogramsper hectare in 2016. Uttar Pradeshhad the largest share (16.4%), followed by Maharashtra (10.8%), Madhya Pradesh (7.6%), Punjab (7%), Andhra Pradesh (6.5%), Karnataka (6.3%), Gujarat (6.2%), Bihar and West Bengal (5.8% each) and Telangana (5.3%). These 10 states accounted for about 78% of the total fertilizer consumption in the country.- A great variability is observed in fertiliser consumption among States from 250 kg / ha in Punjab (Highest), 212 kg / ha in Bihar, 207 kg / ha in Haryana to 4.8 kg / ha in Nagaland and 2 kg /ha in Arunachal Pradesh (lowest), in nutrient form during 2012-13.
- Among UTs it is high in
Pondicherry. - N accounts for 71%, P for 22%, and K for 7% of the total fertilizer consumption.
- Potassic grade of fertilizer is totally imported and is not manufactured in India.
- Urea (85% of N fertilizer consumption) constitutes 58% of the total consumption of fertilizers in India.
- Di-ammonium phosphate (DAP) accounts for approximately 66% of consumption of phosphatic fertilizers.
- The N based fertilizer uses indigenously available feedstock (raw material) to produce ammonia, which is processed further to make urea.
- Rock phosphate and potash, the key raw materials for Phosphatic and Potassic fertilizers respectively are imported into India, due to lack of domestic availability.
Classification of fertilizer:
- Straight fertilizer: Fertilizer which supply
only onemajor plant nutrient e.g. Urea - Complex fertilizer: Fertilizer which supplies
two or moreof the primly nutrients e.g. DAP - Mixed fertilizers: Are the products made by
mixing two or more fertilizerphysically e.g. nitro phosphate with potash 15: 15: 15 of NPK. - Binary Fertilizers: Contains
two nutrients. - Ternary Fertilizers: Contains
three nutrients. - Complete fertilizer: Fertilizer having
all three primary major nutrientse.g. NPK - Low analysis fertilizers: Having
less than 25 %of the primary nutrient e.g. SSP (16% P2O5), sodium nirate (16% N). - High analysis fertilizer: Contains
more than 25 %of the total primary nutrient e.g. Urea.
Fertilizer grade
- Refers to the guaranteed analysis of its plant nutrient.
- Fertilizer grade indicate the percentage of plant nutrients in a fertilizer.
- It is minimum guarantee of the plant nutrient content in the terms of available N, P and K eg. NPK: 19:19:19.
Fertilizer ratio
- Fertilizer ratio is the relative proportion of three major (N, P2O5, K2</subO) plant nutrients.
- Eg. 160 kg fertilizer with ratio of 2:1:1 means 80 Kg N, 40 Kg P2O5 and 40 Kg K2</subO.
Materials used in manufacturing of fertilizers (Mixed)
- Suppliers of plant nutrient: Straight fertilizers are used for this purpose.
- Conditioners: To check absorbing moisture and making one, conditioners like straw, groundnut husk, paddy husk, peat soil etc. are used they just reduce caking and applied in drilling conditions and these conditioners are of low organic materials.
- Neutraliser of acidity or basicity: Dolomitic limestone is used to reduce residual acidity. Most fertilizers leave residual acidity or basicity.
- Filler material or make weight material: Sand, soil, earth, coal ash, charcoal such waste materials are added to make up the difference between the, weight of the added fertilizers required to supply the plant nutrients and the desired quantity of the fertilizer mixture.
Precautions in Mixing fertilizers
- Hygroscopic fertilizers should not be mixed because they form cakes after mixing. Order of hygroscopic nature of fertilizers (decreasing)
Ammonium Nitrate> Urea > Ammonium Sulphate > Ammonium Sulphate Nitrate > CAN
- The most hygroscopic fertilizer is Ammonium nitrate and urea stands second. All these fertilizers have NH4+.
- Fertilizers containing NH4+ should not be mixed with basically reactive materials like lime, basic slag and rock phosphate because mixing results in the loss of N through escape of NH3 gas.
- All water soluble phosphatic fertilizers like super phosphate should not be mixed with those fertilizer that contain free lime because it will convert the soluble phosphate into insoluble form.
- Slightly acidic fertilizer containing chloride may damage the gunny bags and drilling equipments.
Equivalent acidity
- The amount of CaCO3 required to neutralize the acid residue caused by application of acidic fertilizers in the soil.
- E.g. 100 kg (NH4)2SO4 produces acidity which needs 110 kg of CaCO3 to neutralise it.
- Therefore equivalent acidity of (NH4)2SO4 is 110. Anhydrus NH3 has more equivalent acidity than other fertilizer.
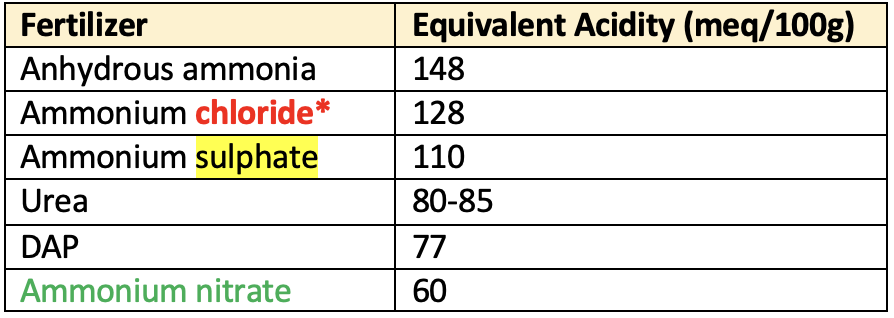
👉🏻 Acidic residual nature of fertilizer
Anhydrous ammonia> Ammonium chloride > Ammonium sulphate > Urea > Ammonium nitrate (lowest)
Equivalent basicity
- Residual basicity caused by application of basic fertilizers, expressed in terms of CaCO3 equivalent of basic residue left by a fertilizer material (in kg/100 kg of fertilizer salt).
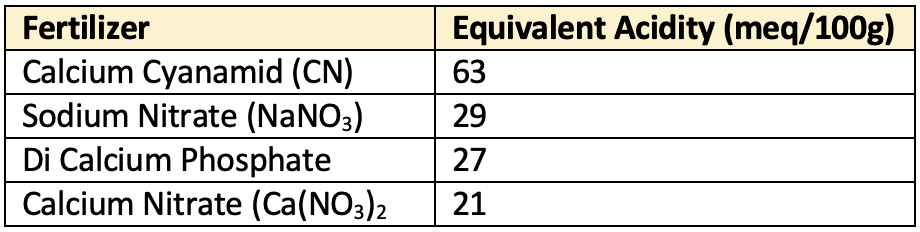
👉🏻 Order of basic residual nature of fertilizer:
Calcium Cyanamid> Sodium Nitrate > Di calcium phosphate > Calcium nitrate
- So these will have zero equivalent acidity.
Fertilizers
- Liebig in Germany and Lawes in England developed independently the idea of treating phosphate with H2SO4 and producing a water-soluble phosphate by about 1840. It is known as superphosphate or simply super.
- Lawes set up a factory at Deptford in 1843 for the production of superphosphate. Possibly this was the first factory to be set up for the production of artificial fertizers on a commercial scale.
- Fertilizers are the organic or inorganic materials of natural or synthetic origin which are added to the soil to supply certain elements essential to the growth of plants.
- Fertilizers are generally inorganic in origin and they supply one/more essential plant nutrients in large proportions.
- The term ‘Fertilizer’ is now commonly restricted to commercial products.
- Amendments are the …
Become Successful With AgriDots
Learn the essential skills for getting a seat in the Exam with
🦄 You are a pro member!
Only use this page if purchasing a gift or enterprise account
Plan
Rs
- Unlimited access to PRO courses
- Quizzes with hand-picked meme prizes
- Invite to private Discord chat
- Free Sticker emailed
Lifetime
Rs
1,499
once
- All PRO-tier benefits
- Single payment, lifetime access
- 4,200 bonus xp points
- Next Level
T-shirt shipped worldwide

Yo! You just found a 20% discount using 👉 EASTEREGG

High-quality fitted cotton shirt produced by Next Level Apparel