🚶 Nitrogenous Fertilizers
Classification, Controlled Release
- The ultimate source of nitrogen is atmosphere and immediate and important source of nitrogen is organic matter.
- Except paddy and potato, the ammonical form is not directly absorbed by crops. Ammonia always produces acidity. Except NO3-, CaCN and CAN; all other nitrogenous fertilizers are acidic in nature. NO3- and CaCN are basic in reaction, whereas CAN is neutral.
- Maximum consumption of N & P in the state: Uttar Pradesh
Classification of Nitrogen Fertilizers
Nitrate - N fertilizer
- Highly mobile in soil.
- Suitable for top dressing.
- Nitrate fertilizers are basic residual in nature.
- Highly soluble and subjected to leaching and denitrification in water logged soils more suitable for dry soils.
Examples of nitrate fertilizer
- Sodium Nitrate:
16% N, Chilean nitrate, pioneer nitrogenous fertilizer; useful for acidic soils. Its continuous and abundant use causes deflocculation and develop a bad physical condition in low rainfall regions. - Calcium Nitrate:
15.5% N - Potassium Nitrate (KNO3):
13 % Nand 46 % K2O
Ammoniacal - N fertilizer
- Less leaching as NH4+ is adsorbed on clay complex, but readily soluble in soil.
- Suitable for water logged soils.
- Acidic residual nature.
- Volatilization & Denitrification losses more.
Examples of ammoniacal fertilizer
a. Ammonium sulphate ((NH4)2SO4)

- Contains
20.6 % Nand24.5 % Sulphur. (Highest sulphur found) UPPSC 2021 - Most suitable for paddy, tea, groundnut & sugarcane
- For rice crop: ammonium sulphate is the best.⭐️
- In water logged soils ammonium sulphate is best suitable N fertilizer.
- It is a white crystalline salt and to some extent hygroscopic.
- It is very soluble in water and so called as quick acting fertiliser.
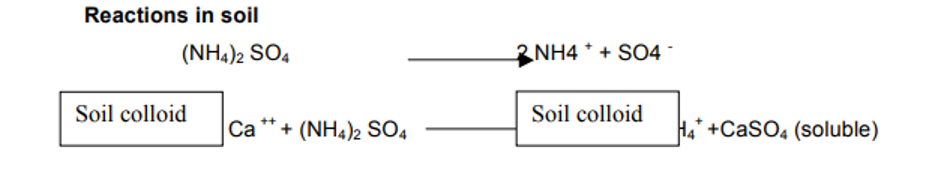
- During fixation, NH4+ releases an equivalent quantity of Ca2+ from the soil and CaSO4, that results, is leached away under humid conditions.
- Application of (NH4)2SO4 reduces the quantity of reserved calcium in soil. If there is no reserve of Ca in the soil, the H2SO4 is formed as a residual product which will increase the acidity in soil.
b. Ammonium Chloride (NH4Cl)

- It contains
25.5 % N - Being acidic residual nature it is extensively used in paddy in Japan.
- It is usually not recommended for tomatoes, tobacco etc.
- Cereal crops are not affected by Cl- as most of it is retained by straw and is not translocated to the grain.
- In some crops like potato and sweet potato high quantity of Cl- is avoided and instead K2SO4, KNO3 is used (KNO3: 13.85% N and 46-47% K2O).
c. Aqueous ammonia
80% N, used as fertigation i.e. in irrigation water.
NH 4+ and NO3- N fertilizer
- NO 3 - nitrogen readily available to plants for rapid growth and NH4+ nitrogen at the later stage:
(i) Ammonium nitrate (NH4 NO3)

- It contains
33 % N(half of ammonium form and half nitrate) - Highly hygroscopic nature (Highest)
- Explosive fertilizer IBPS AFO 2012
(ii) Calcium ammonium nitrate (CAN)

- Commonly known as Kishan Khad.
- It contains
26% N. (half of N is in ammoniacal form). - Neutral in nature.
- Most suitable for vegetables.
- Commercially prepared from amm. nitrate and ground limestone.
- According to standard, moisture content by wt. < 1%, calcium nitrate < 0.5% by weight.
Amide fertilizer
- Organic fertilizer, first converted into ammonical and then to nitrate form.
- Urea is acidic but CaCN is basic.
- Highly soluble in water.
Amide → (NH4)2CO3 → NO3-
Organic form: Urea (NH2 CO NH2)

- The CO2 and NH3 are allowed to react in the liquid phase under greatly elevated pressure and temperature and this process requires highly specialized equipment.
- Most commonly used fertilizer in India
- Cheapest source of nitrogen.
- Contains
46 % N - It is hygroscopic therefore produced in granular/pellet form and is coated with a non-hygroscopic inert material.
- Max. moisture:
1 %by wt. and Biuret:< 1.5 %according to fertilizer control order. - When urea will be applied as foliar spray on crop canopy the biuret concentration should not exceed 0.25%.
- The concentration of foliar spray of urea varies between 2-6% but general concentration is 2 %.
- Biuret is formed during manufacturing of urea when temp, goes high above 150°C and is toxic to plants when its concentration is more than 2%.

- Acidic in residual: continuous use of urea for several years results reduce soil pH.
- Neem coated urea: being slow release properties it increases the nitrogen use efficiency.

- Urea in the presence of ureas forms into Ammonium Carbamates.
- When urea is applied to the soil, following reactions takes place.
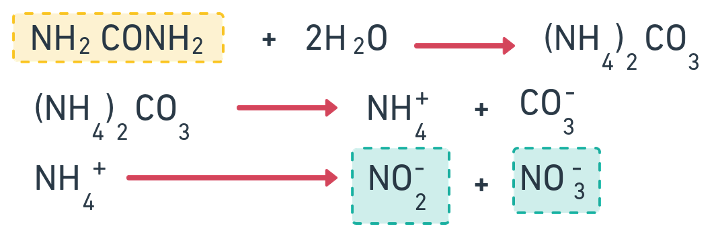
- The whole conversion takes about 4-7 days. Therefore, it is advisable to apply Urea 3-4 days before sowing.
Inorganic form
Calcium cyanamide

- Contains
21 % N - Certain intermediary compounds formed during its decomposition injure tender and germinating seedlings and therefore it is advisable to apply it at least a week before sowing. Lime is one of the products of decomposition and a certain amount of lime is also present in the original material and these are of high value in acid soils.
- Neither import or nor manufactured in India.
Nitrification Inhibitors and Slow-release Fertilizers
- Among the major nutrients, P & K are less mobile as compared to N. Nitrogenous fertilizers are easily lost by leaching, volatilization and denitrification. To offset these losses to the minimum, N is applied in split doses at the critical phases of crop growth. However split application increases the cost of fertilizer application. In order to reduce the leaching, volatilization and denitrification losses to the minimum, use of some nitrification inhibitors or slow-release fertilizers are useful to regulate the nutrient availability to the crop.
👉🏻 Advantages of Nitrification Inhibitors and slow-release fertilizer
- They improve the uptake of N by releasing the nutrient slowly and uniformly.
- Labour saving device since no need for split application.
- Scope for reducing the fertilizer dose due to higher efficiency of fertilizer and uptake of nutrient.
- Low pollution of water and air since leaching losses are minimum.
- Application of fertilizer can be done either, basal or top dressing thereby greater flexibility in timing.
Nitrification Inhibitors
- Inhibitors are used with good results in case of non-nitrate fertilizers such as ammonia, ammonium salt, urea etc.
- Inhibitors decrease the activity of nitrifying bacteria e.g.
- For lowland:
- Oxamide (NH2CO-CONH2): 31% N, not hygroscopic, solubility is 0.4g/lit. water.
- Dicyandiamide (DD): NH2C (=NH) NHCN: 42% N
Thiourea(TU): 36.8% N- Urea pyrolyzate: 48% N
- For upland:
- AM (2-amino-4-chloro-6-methyl pyrimidine)
- N-Serve (2-chloro-6-trichloromethyl pyridine)
- Others:
- ASU (Guanyl thiourea)
- Nitrapyrin
- ST (Sulphathiazole)
- DCS
- ATC
Neem Cake
Slow Release fertilizers RRB-SO-2020
- To overcome the problem of leaching, the solubility of nitrogen fertilizers are reduced by
👉🏻 (A) Synthesizing compounds which are inherently less soluble e.g.
- lsobutylidene diurea (IBDU): 31-32 % N
- Crotonylidene diurea (CDU): 32.5 % N
- Guanyl urea sulphate (GUS)
- Urea Formaldehyde (UF/Urea Form): 38-42 % N, less hygroscopic than urea
- Oxamide
👉🏻 (B) Coating barriers to the presently available fertilizers e.g.
- Sulphur coated urea
- Sulfonyl-urea
- Neem coated urea
- Lac coated or shellac coated urea (34.2% N)
👉🏻 (C) Formation of super Granules/Modified form: Big granules of urea 1-4 g each (Palletization) are made. When these granules are placed in the reduced zone of soil, the losses are substantially reduced. To facilitate deeper placement, Urea is manufactured as super granules, briquetts or mixed with mud and made into balls.
- GROMOR/Gromor: Trade name of urea-ammonium phosphate grade: 29 : 29 : 0
- Ammophos-B: Earlier name of Ammonium phosphate sulphate, grade: 20 : 20 : 0
Neem Coated Urea (NCU)
- In January 2015, the Government allowed the urea produce upto 100 per cent of production as Neem coated urea. Further, the Government made it mandatory to produce at least 75 per cent of domestic urea as Neem coated. The current policy is that Government has mandated all indigenous producers of urea to produce 100 per cent of urea as Neem coated urea only.
- The scheme being promoted to regulation the use of urea, enhance the availability of nitrogen to the crop and reduce to cost of fertilizer consumption.
- Urea coated with Neem oil is known as Neem coated urea. Only about 30 to 40 per cent of N2 in the urea is utilized by the plants. Coating of Neem oil helps in gradual release of nitrates into soil. This may work as a biopesticide.
- Primary motive of the Indian government is to stop the illegal diverting of highly subsidized urea for the industrial purpose. Neem coated urea is unfit for industrial purpose.
Controlled-Release Fertilizers Using Zeolites
- The U.S. Geological Survey (USGS) has experimented with zeolites to help control the release of fertilizer nutrients in soil. The use of soluble fertilizers can lead to water pollution and to wasted nutrients. Nitrogen, for example, can leach into ground and surface waters, especially in sandy soils, and phosphate may become fixed and unavailable to plants, especially in tropical soils.
- Zeolites are porous minerals with high cation-exchange capacity that can help control the release of plant nutrients in agricultural systems. Zeolites can free soluble plant nutrients already in soil, and may improve soil fertility and water retention. Because zeolites are common, these unique minerals could be useful on a large-scale in agriculture.
- The ultimate source of nitrogen is atmosphere and immediate and important source of nitrogen is organic matter.
- Except paddy and potato, the ammonical form is not directly absorbed by crops. Ammonia always produces acidity. Except NO3-, CaCN and CAN; all other nitrogenous fertilizers are acidic in nature. NO3- and CaCN are basic in reaction, whereas CAN is neutral.
- Maximum consumption of N & P in the state: Uttar Pradesh
Classification of Nitrogen Fertilizers
Nitrate - N fertilizer
- Highly mobile in soil.
- Suitable for top dressing.
- Nitrate fertilizers are basic residual in nature.
- Highly soluble and subjected to leaching and denitrification in water logged soils more suitable for dry soils.
Examples of nitrate fertilizer
- Sodium Nitrate:
16% N, Chilean nitrate, pioneer …
Become Successful With AgriDots
Learn the essential skills for getting a seat in the Exam with
🦄 You are a pro member!
Only use this page if purchasing a gift or enterprise account
Plan
- Unlimited access to PRO courses
- Quizzes with hand-picked meme prizes
- Invite to private Discord chat
- Free Sticker emailed
Lifetime
- All PRO-tier benefits
- Single payment, lifetime access
- 4,200 bonus xp points
- Next Level
T-shirt shipped worldwide

Yo! You just found a 20% discount using 👉 EASTEREGG

High-quality fitted cotton shirt produced by Next Level Apparel