☢️ Problem Soils
Secondary Nutrients, Micronutrients, Methods of application, Suitability, Recommendation, NBS
- The phenomenon of accumulation of excess salt/acid in the root zone, results in a partial or complete loss of soil productivity and such soil is defined as ‘Problem Soils’ (Alkali, Saline & Acid) and exist mainly in arid and semi-arid regions.
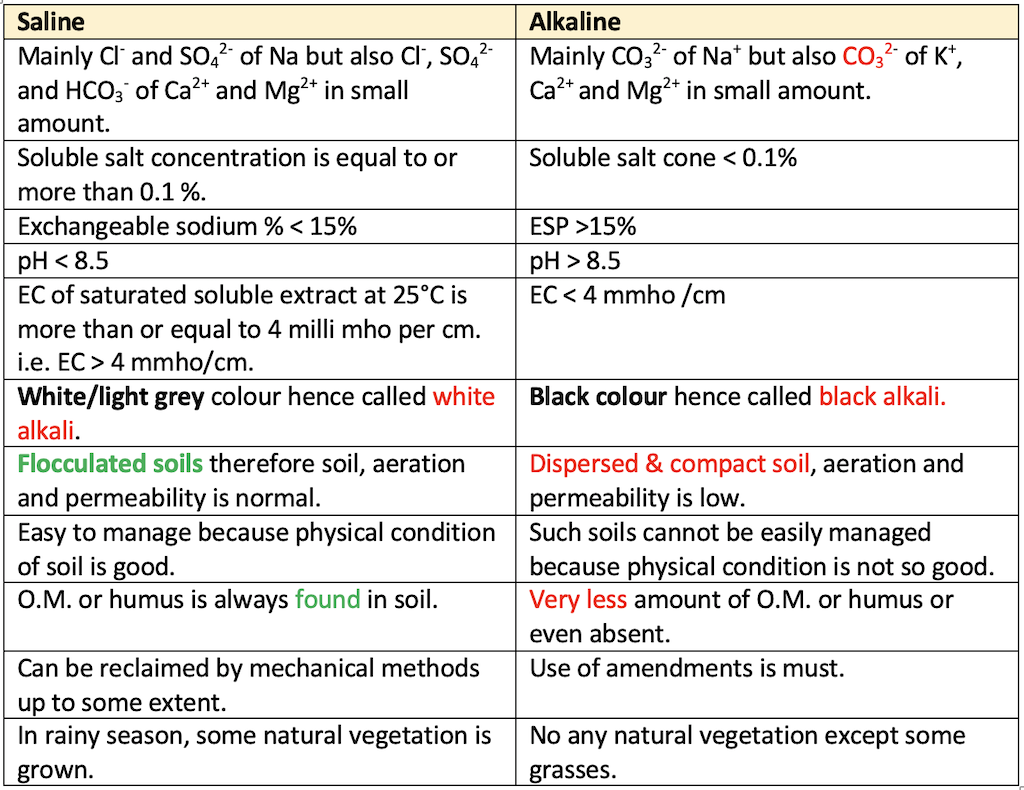
- When the Chloride (Cl-), Sulphate (SO42-), Carbonate (CO32-) and Bicarbonate (HCO3-) salts of Sodium (Na+), Calcium (Ca2+) and Magnesium (Mg2+) are increased in Soil; the soil becomes saline and alkaline. On the basis of amount of soluble salts, average quantity of exchangeable sodium and pH, such soils are classified as saline soil, alkali soil and saline-alkali soil.
Saline Soils

- When the Chloride (Cl-), Sulphate (SO42-) salts of Sodium (Na+), Calcium (Ca2+) and Magnesium (Mg2+) are increased in Soil with soils having a conductivity of the saturation extract
greater than 4 dSm-1 IBPS 2018 and an exchangeable sodium percentageless than 15. - The pH is usually
less than 8.5. - Formerly these soils were called
white alkali soilsbecause of surface crust of white salts. - Also called
Solonchack. - Two types of saline soils:
- Having substantial quantity of Ca and Mg.
- Having chiefly Na and damages the colloidal complex.
Genesis
- Most serious salinity problem is being faced in those arid and semi-arid regions of the country, where canal irrigation is major source of irrigation.
- The process by which the saline soil formed is called
salinization. Saline soils occur mostly in arid or semi-arid regions. - Salt affected soils are also found extensively in sub-humid and humid climates, particularly in the coastal regions where the ingress of sea water through estuaries & rivers causes large-scale salinization.
Favourable Conditions
- In arid regions saline soils occur not only because there is less rainfall available to leach and transport the salts but also because of high evaporation rates & high temperature which tend further to concentrate the salts in soils and in surface waters.
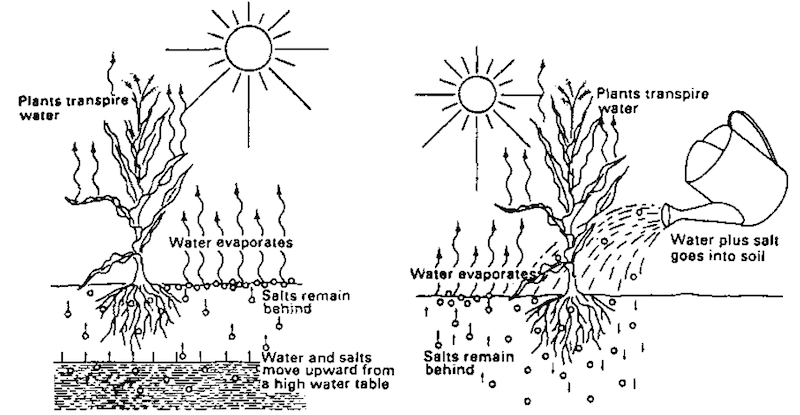
Major production constraints
- Presence of salts leads to alteration of osmotic potential of the soil solution.
- Consequently water intake by plants restricted and thereby nutrients uptake by plants are also reduced.
- In this soil due to high salt levels microbial activity is reduced.
- Specific ion effects on plants are also seen due to toxicity of ions like chloride, sulphate, etc.
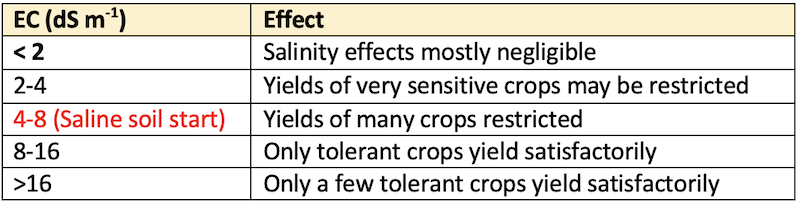
Electrical Conductivity of the soil saturation extract
- Measurement of EC of the soil saturation extract is essential for the assessment of saline soil for the plant growth.
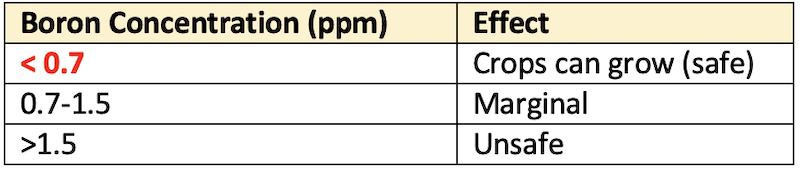
Concentration of water-soluble boron
- The determination of water-soluble boron concentration is also another criterion for characterization of saline soils. The critical limits of boron concentration for the plant growth are given below.
Reclamation
- Principle: Removal of excess salts to a desired level in the root zone.
- This can be done either
- By scraping the salts from the surface
- Washing them down into lower layers beyond the root zone preferably completely out of the solum
- By growing salt tolerant crops (or) by a combination of two (or) more of these methods
- Scraping helps to remove salts that have formed an encrustation on the surface, but it is never very helpful in complete reclamation. Substantial quantities of soluble salts are still present in the soil body and hinder plant growth.
Management of saline soils
- The reclamation of saline soils involves basically the removal of salts from the saline soil through the processes of leaching with good quality water and drainage.
- Provision of lateral and main drainage channels of 60 cm deep and 45 cm wide and leaching of salts could reclaim the soils. Therefore, provision of adequate drainage system is a pre-requisite for any reclamation process.
- Sub-surface drainage is an effective tool for lowering the water table, removal of excess salts and prevention of secondary salinisation.
Irrigation management
Leaching with water of good qualityandadequate drainageare two essential components of any permanent solution of the salinity problem.- Ponding of water is the most commonly used for leaching. Intermittent ponding is more efficient than continuous ponding.
Ricecan be grown during initial years of reclamation.
Bio drainage
- Soil salinity is also a serious problem in areas where groundwater with high salinity is used for irrigation.
- Five-year-old tree of Eucalyptus could lower the water table by 85 cm with an average transpiration rate 50 litter per day per plant.
Fertilizer management
- Addition of extra dose of nitrogen to the tune of 20-25% (because of alkalization losses) of recommended level will compensate the low availability of N in these soils.
- Addition of organic manures like, FYM, compost, etc. helps in reducing the ill effect of salinity due to release of organic acids produced during decomposition.
- Green manuring (Sunhemp, Daincha, Kolingi) and or green leaf manuring also counteracts the effects of salinity.
- Application of farm yard manure at 5 t ha-1 at 10-15 days before transplanting in the case of paddy crop and before sowing in the case of garden land crops can alleviate the problems of salinity. Crop choice / Crop management Crops are to be chosen based on the soil salinity level.
Soil / Cultural management
- Planting the seed in the centre of the raised bed / ridge may affect the germination as it is the spot of greatest salt accumulation.
- A better salinity control can be achieved by using sloping beds with seeds planted on the sloping side just above the water line.
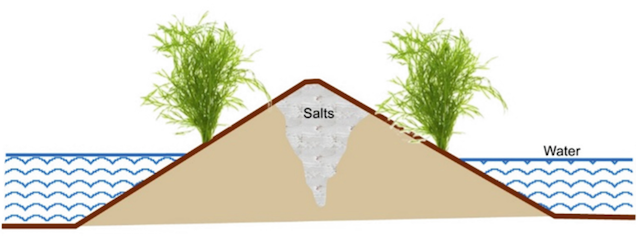
- Alternate furrow irrigation is advantageous as the salts can be displaced beyond the single seed row.
- Application of straw and polythene mulch had been found to curtail the evaporation from soil surface resulting in the reduced salt concentration in the root zone profile within 30 days.
- Management of saline soils becomes essential and unavoidable particularly in areas where both soil as well as irrigation water is saline in nature.
Salt tolerant crops
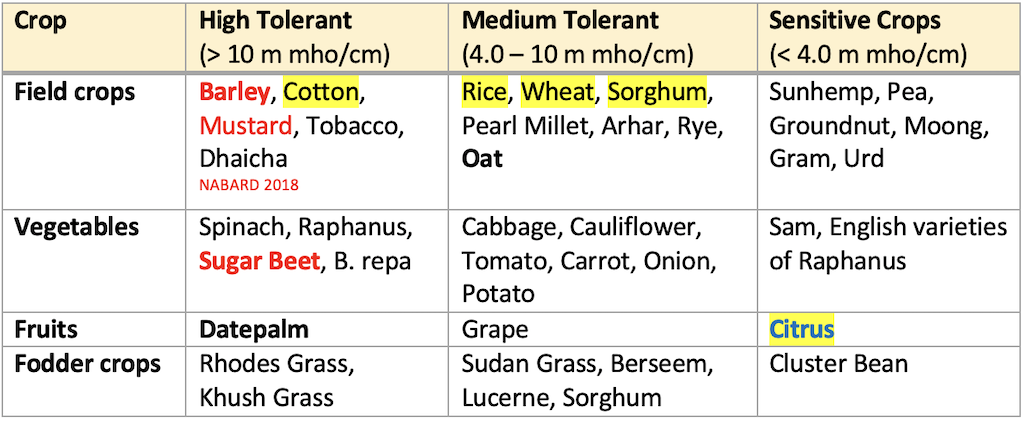
- The growing of salt tolerant plants with a view to remove salts is also not a practical proposition.
- Although these plants remove substantial quantities of salts from the soil, comparatively larger quantities are still left behind. Salt formation is a continuous process; hence, the reclamation is never complete.
Occurrence in India
- The saline soils are found mainly in the States of
Gujarat, West Bengal, Odisha, Rajasthan, Bihar, Haryana, Maharashtra, Andhra Pradesh, Kerala, Tamil Nadu, Uttar Pradesh. - Isolated patches of problem soils are also found in other States.
Alkali Soil (Sodic/ Solonetz)

- Alkali (or)
Sodic Soilis defined as a soil having a conductivity of the saturation extract less than 4 dS m-1 and - An
ESP of > 15 - The pH is usually between
8.5 and 10.0. (> 8.5 AFO 2017/2018) - Low infiltration rate.
- Formerly these soils were called
black alkali soils. - Found in semi-arid and sub-humid areas.
Genesis
- Calcium and Magnesium are the principal cations found in the soil solution and on the exchange complex of normal soils in arid regions.
- When excess soluble salts accumulate in these soils, sodium frequently becomes the dominant cation in the soil solution.
- In arid regions as the solution becomes concentrated through evaporation or water absorption by plants, the Ca2+ and Mg2+ are precipitated as CaSO4, CaCO3 and MgCO3 with a corresponding increase in sodium concentration.
- When the Na+ concentration is
more than 15 %of the total cations a part of the original exchangeable Ca2+ and Mg2+ replaced by sodium resulting in alkali soils.
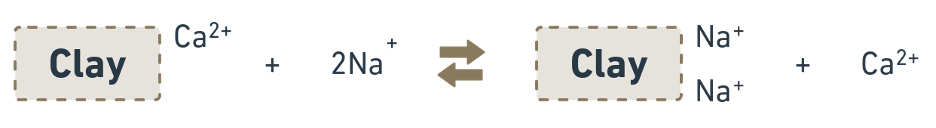
- Though the reaction is reversible, Ca2+ are removed in drainage water as soon as they formed. Hence, the reaction proceeds in one direction from left to right only. The process whereby a normal soil is converted into an alkali soil is known as “
alkalization”. - There are three distinct stages in the evolution of alkali soils viz.
- Salination
- Saline-Alkaline soil
- Alkalization i.e. desalination and intense alkali soil formation
Characteristics
- A direct determination of exchangeable sodium:
Exchangeable Sodium = Total Sodium - Soluble Sodium
- The soil pH also gives an indication of soil alkalinity indirectly. An increase in pH reading of 1.0 or more, with change in moisture content from a low to high value has itself been found useful in some area for detecting alkali conditions.
- The higher the ESP, the higher is the soil pH.
- Sodium Adsorption Ratio (SAR)
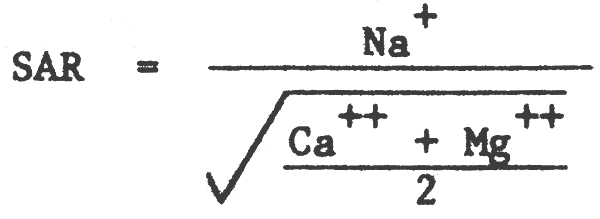
- The US Salinity Laboratory developed the concept of SAR to define the equilibrium between soluble and exchangeable cations (Na+, Ca2+, Mg2+ are concentrations in saturation extract in me L-1).
- The value of SAR can be used for the determination of Exchangeable Sodium Percentage (ESP).
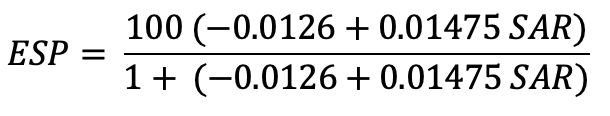
Impact of soil sodicity
- Dispersion of soil colloids leads to development of
compact soil.
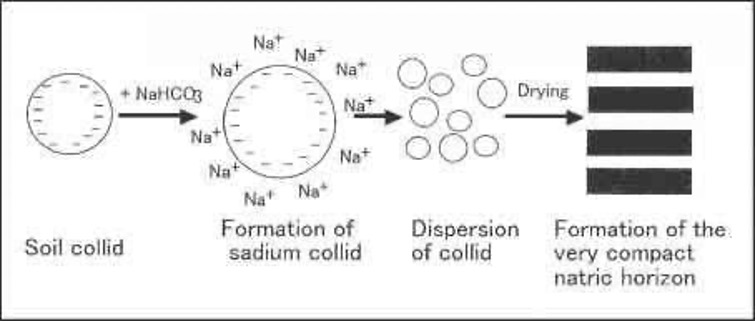
- Due to compactness of soil, aeration, hydraulic conductivity, drainage and microbial activity are reduced.
- High sodicity caused by Na2CO3 increases soil pH.
- High hydroxyl (OH-) ion concentration has direct detrimental effect on plants.
- Excess of Na+ induces the deficiencies of Ca2+ and Mg2+.
- High pH in alkali soil decreases the availability of many plant nutrients like N, P, Ca, Fe, Mg, Cu, and Zn.
Reclamation of alkali / sodic soils
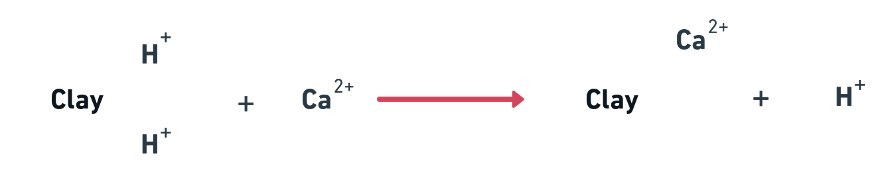
- Basic Principle: To replace exchangeable Na+ by Ca2+ and thus released Na+ salt be leached out of root zone. The following amendments and practices are:
- Gypsum (CaSO4. 2H2O) AFO-2021 NABARD 2020
- Iron pyrite (FeS2)
- Several other amendments like: Calcium Salts, Acids, Acid forming materials. The choice of amendment depends upon the nature and quantity of CO32- present in soils.
- Bulky Organic manures, green manures, crop residues and others produce weak organic acids. These are applied in conjugation with Gypsum.
- Leaching with water of good quality.
Management Practices
- Cropping with green manure.
- Rice-Dhaincha in U.P.
- Dhaincha-Rice-Berseem in Punjab.
- Higher dose of N because of volatilization. Application of zinc in initial years of reclamation.
- Frequent irrigation with small quantities of water.
Crop choice
Riceis preferred as first crop in alkali / sodic soil as it can grow under submergence, can tolerate fair extent of ESP and can influence several microbial processes in the soil.
Other measures
- Agroforestry
- Soil should be removed after digging the small plots and refilled it with pond soil and dung.
- Grow jungle of Acacia, which reclaims it in 15-20 years because tap root breaks the hardpan.
Occurrence in India
- The alkali soils are largely predominant in the Indo-Gangetic plains encompassing States of
GujaratAFO 2018, Uttar Pradesh, Punjab, Haryana & Bihar and partly in states like, Chhattisgarh, Rajasthan, Andhra Pradesh, Maharashtra, Karnataka, Andhra Pradesh, Madhya Pradesh and Tamil Nadu.
👉🏻 Table: Difference between saline and alkaline Soils
Saline-Alkali/ Saline-Sodic Soils
- Saline-Alkali / Sodic Soil is defined as a soil having a conductivity of the saturation extract
greater than 4dS m-1 and an exchangeable sodium percentage (ESP)greater than 15. AFO 2018 - The pH is variable and usually
above 8.5depending on the relative amounts of exchangeable sodium and soluble salts. - When soils dominated by exchangeable sodium, the pH will be more than 8.5 and when soils dominated by soluble salts, the pH will be less than 8.5.
Formation
👉🏻 Favorable factors are:
- Aridity
- Poor external or internal drainage
- Irrigation by salty water
- Permanent water courses
- Rise in water table by excess irrigatio
- Erratic use of irrigation water i.e. flooding followed by intense drought.
- Saline-alkali soil is difficult to manage since its physical condition is very bad.
Management
- The reclamation / management practices recommended for the reclamation of sodic soil can be followed for the management of saline – sodic soil.
Detrimental effect of soil salinity and alkalinity
- Soil structure: in alkali condition, soil is dispersed and becomes compact.
- Less water permeability due to compactness.
- Low aeration
- Low microbial activities.
- Unavailability of nutrients like P, Ca, N
- Nutritional disturbances at pH 8.5 and more.
- Hindrance in water absorption.
- Effects of osmotic pressure: Increase in osmotic pressure of soil solution badly affects the plant’s growth.
- Salt toxicity: In alkali soil, Na2CO3 is highly toxic.
Acidic Soils

- Soil acidity refers to presence of higher concentration of H+ concentration in soil solution and at exchange sites.
- Soil acidity is a major problem in relation to plant growth and therefore acid soils are called a problem soil.
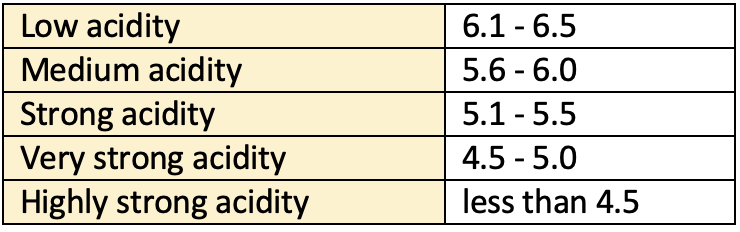
- Acid soils are characterized by low soil pH, which varies form strongly acidic (4.5-5.5) to extremely acidic (4.5) and with low base saturation.
Soil acidity and pH Soil acidity pH
- Soil acidity is of three kinds viz., active acidity, exchangeable acidity and residual acidity.
- The hydrogen ions in the soil solution contribute to active acidity. It may be defined as the acidity developed due to concentration of H+ and Al3+ ions in the soil solution. Kaolinite and Illite clay minerals are dominant.
- The concentration of hydrogen ion in soil solution due to active acidity is very small, implying that only a meager amount of lime would be required to neutralize active acidity. Inspite of smaller concentration, active acidity is important since the plant root and the microbes around the rhizosphere are influenced by it.
- The concentration of exchangeable Al3+ and H+ ions contribute to exchangeable acidity. It may be defined as the acidity developed due to adsorbed H+ and Al3+ ions on soil colloids.
- Aluminum hydroxyl ions and H+ and Al3+ ions present in non-exchangeable form with organic matter and clay account for the residual acidity.
Total acidity = Active acidity + Exchangeable acidity + Residual acidity
Sources of soil acidity
-
Leaching due to heavy rainfall
- Acid soils are common in all regions where rainfall or precipitation is high enough to leach appreciable amounts of exchangeable bases from the surface soils and relatively insoluble compounds of Al and Fe remains in soil.
- The nature of these compounds is acidic and its oxides and hydroxides react with water and release hydrogen ions in soil solution and become acidic. Besides, when the soluble bases are lost, the H+ ions of the carbonic acid and other acids developed in the soil replace the basic cations of the colloidal complex. As the soil gets gradually depletes of its exchangeable bases through constant leaching, it gets desaturated and becomes increasingly acid.
CO2 + H2O ⇋ H2CO3 H2CO3 + CaCO3 ⇋ Ca(HCO3)2 (Leachable)
-
Acidic parent material
-
Acid forming fertilizers and soluble salts: Use of ammonium sulphate and ammonium nitrate increases soil acidity
Processes involved in acid soil formation
- Laterization of varying degrees.
- Podzolization with sub-temperate to temperate climate.
- Intense leaching of light alluvial soils.
- Marshy conditions with significant amount of partly decomposed O.M.
Characteristics of acid soils
[A] Physical:
- Light texture
- High permeability
- Poor water holding capacity
- Poor cation exchange capacity
- Poor O.M. content
- Mainly kaolinite and sometimes illite clay minerals
[B] Chemical:
- Base unsaturated soil, more anions than cations.
- Active and potential soil acidity.
- Toxic effects on plants: Al3+ Conc. more.
- Availability of nutrients at
👉🏻 Table: Soil pH and nutrients availability
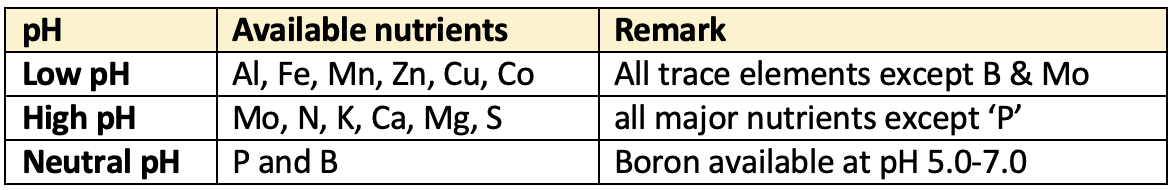
[C] Biological:
- Population of Fungi more than that of bacteria. There is no effect of pH on the population of fungi.
- Fungi cause diseases.
- Rate of decomposition of biological materials and rate of mineralisation and nitrification etc. are reduced when acidity is increased.
Management of acid soils:
- In two ways –
- Either by growing crops suitable for particular soil pH or
- By ameliorating the soils through the application of amendments.
- The former is rather risky. (Intensive and continuous cropping of arable crops in humid regions will aggravate soil acidity and use of acid producing fertilizers will further accentuate the process. Acid soils are made more suitable for agricultural use by liming which raises the soil pH.
- Liming is generally recommended for soils with pH less than 5.5.
Liming Materials
- Calcium limestone (CaCO3). More than 90% use in India.
- Dolomite (rich in Mg)
- Quick lime (CaO)
- Slaked lime Ca(OH)2
- Coral shell lime
- Chalk CaCO3
- Blast furnace slag CaSiO3 and Ca2SiO4
- Miscellaneous sources like wood ashes etc.
Lime Requirement
- High lime requirement: Barley, Beans, Cotton, Pea, Soybean, Sugarbeet and Sunflower.
- Medium lime requirement: Corn (Maize), Sorghum, Tobacco, Wheat.
- Low lime required: Oat, Potato, Rice, Rye.
- Very low lime required:
Pine apple.
Crop choice
- Selection of crops tolerant to acidity is an effective tool to counter this soil problem and breeding of such varieties is of specific importance for attaining higher productivity, particularly in areas where liming is not an economic proposition. The crops can be grouped on the basis of their performance in different soil pH range.
- Relative tolerance of crops to soil acidity.
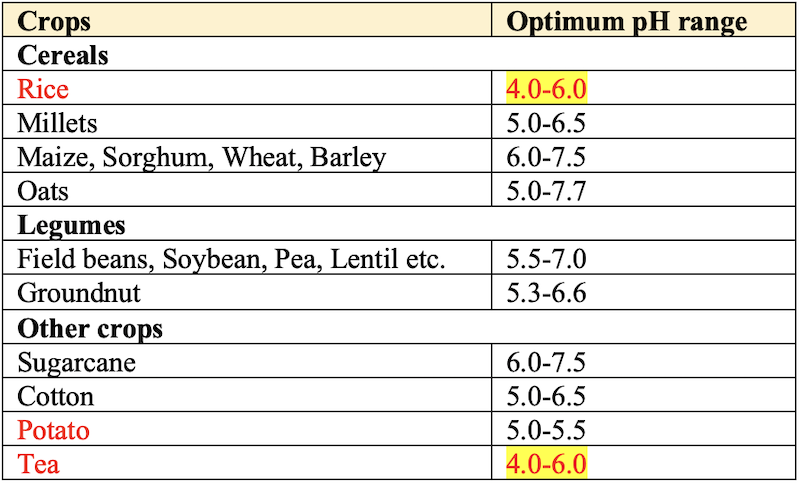
Occurrence in India
- The problem of acid soils exists in most of the States except Gujarat, Rajasthan, Punjab and Uttar Pradesh.
- Mostly acidic soils found in
hilly areas. AFO 2017 - Highest Percentage: 95% of soils of Assam.
- Highest Area:
Kerala> Chhattisgarh - About 80% of soils in Orissa, 88% in Kerala, 45% in Karnataka and 20% in Maharashtra are acidic.
👉🏻 Total Problem soils: 24.36 million ha (17.93 Acidic + 3.7 Alkaline + 2.73 Saline)
🔥 Highest Saline Soil: Gujarat > WB (Highest Coastal Saline Soil) UPPSC 2021
🔥 Highest Alkali Soil: UP > Gujarat
🔥 Highest Acidic Soil: Kerala > Chhattisgarh
Government Schemes
Scheme for Reclamation of Problem Soils-as sub-scheme of RKVY
- Upper limit of unit cost will be
Rs. 60,000 per hafor alkaline/saline soils andRs. 15,000 per hain case of acid soil for reclamation on Projected Approach basis or actual whichever is less. AFO - Funding pattern between the Centre and the State Governments respectively for all states except eight north-eastern states and 3 Himalayan states under the RKVY:
- 60: 40. For North Eastern and Himalayan states it is 90:10.
National Mission for Sustainable Agriculture (NMSA): Soil Health Management Component
- The assistance provided under National Mission for Sustainable Agriculture (NMSA):
- For the reclamation of the problematic soils (alkaline/saline):
- Alkaline/Saline: 50% of cost to a limit of Rs. 25,000 per ha and or Rs. 50,000 per beneficiary.
- Acidic Soil: 50% of cost to a limit of Rs. 3,000 per ha and or Rs. 6,000 per beneficiary.
References
- Text book of Soil Science by T.D. Biswas and S.K. Mukherjee. Tata McGraw-Hill Publishing Company Limited, New Delhi.
- A text book of Soil Science by D.K. Das. Kalyani Publishers
- Acid Soils of India and Liming by Mandal, S.C., M.K. Sinha and H. Sinha. 1975. Tech. Bulletin (Agric), 51; ICAR, New Delhi.
- Soil Acidity and liming by Adams, F. (Ed) (1984). 2nd Edn, American Society of Agronomy, Madison, U.S.A.
- The phenomenon of accumulation of excess salt/acid in the root zone, results in a partial or complete loss of soil productivity and such soil is defined as ‘Problem Soils’ (Alkali, Saline & Acid) and exist mainly in arid and semi-arid regions.
- When the Chloride (Cl-), Sulphate (SO42-), Carbonate (CO32-) and Bicarbonate (HCO3-) salts of Sodium (Na+), Calcium (Ca2+) and Magnesium (Mg2+) are increased in Soil; the soil becomes saline and alkaline. On the basis of amount of soluble salts, average quantity of exchangeable sodium and pH, such soils are classified as saline soil, alkali soil and saline-alkali soil.
Saline Soils

- When the Chloride (Cl-), Sulphate (SO42-) salts of Sodium (Na+), Calcium (Ca2+) and Magnesium (Mg2+) are increased in Soil with soils having a conductivity of …
Become Successful With AgriDots
Learn the essential skills for getting a seat in the Exam with
🦄 You are a pro member!
Only use this page if purchasing a gift or enterprise account
Plan
- Unlimited access to PRO courses
- Quizzes with hand-picked meme prizes
- Invite to private Discord chat
- Free Sticker emailed
Lifetime
- All PRO-tier benefits
- Single payment, lifetime access
- 4,200 bonus xp points
- Next Level
T-shirt shipped worldwide

Yo! You just found a 20% discount using 👉 EASTEREGG

High-quality fitted cotton shirt produced by Next Level Apparel