👛 Nitrogen
Forms, Loss, Cycle, Fixation, Function, Deficiency, Toxicity
- Soil Weight (i.e. furrow slice) = 2 x 106 kg/ha.
- On an average total nitrogen status of soil is 0.03 – 0.05% N means 1000 kg of N/ha.
- The cheap source of N is the crop residues in temperate region.
- In tropical soils, the total N (Available + Fixed) content is 0.03 - 0.1 %.
- 20-40% of total surface soil N is in the form of bound amino acids; 5-10% as combined hexose amines. Usually only 1-3% of the total amount of N present is mineralised during growing season.
- Rainfall is also source of N at 4.6 kg of N/ha is received ha-1 yr-1. It is converted to NO3- during lighting. Addition of organic matter and fertilizer is other major sources.
- Nitrogen is an essential constituent of protein and also other non-protein compounds of great physiological importance in plant metabolism.
- It is an integral part of chlorophyll, which is primary observer of light energy needed for photosynthesis.
- Nitrogen also imparts vigorous vegetative growth and governs the utilization of P, K and other elements.
Forms of soil nitrogen
- Plant absorbs N as both - NH4+, NO3-
- Inorganic forms
- Ammonium NH4+
- Nitrate NO3
- Nitrite NO2
- Organic forms: Amide form (NH2)
- Elemental N
Losses of Nitrogen
- Crop removal (After Cereals – Grow Pulses)
- Leaching (or) drainage (11 - 18% loss)
- Gaseous losses as NH4 or elemental N2
- Volatilization
- Erosion (8 – 15 kg ha-1 yr-1)
- Ammonia fixation by clays
- Immobilization in organic materials
N transformations in soils
- N Mineralisation
- Aminisation
- Conversation of urea
- N Immobilization
- N factor
- Ammonification
- Nitrification
- Denitrification
- Organic fixation
- Elemental N loss
- Nitrogen cycle
Nitrogen immobilization
“Immobilization is the process of conversion of inorganic N (NH4+ or NO3-) to organic N and it is basically the reverse of N mineralization”.
- The Microorganisms accumulate NH4+ - N and NO3 – N in the form of protein, nucleic acid and other complexes.
- If C : N ratio is wider than 30, it favors immobilization and lesser C:N ratio encourage mineralization.
- C: N ratio > 30:1 = Net Immobilization
- C: N ratio < 20:1 = Net Mineralization
- C: N ratio = 15-30 both immobilization and mineralization
Nitrogen mineralization
- Mineralization is the conversation of organic N to NH4+ as a result of microbial decomposition.
- Mineralization increases with a rise in temperature and is enhanced by adequate, although not excessive, soil moisture and a good supply of O2.
- Mineralization of organic N involves in two reactions i.e.
- Aminisation
- Ammonification
Aminisation
- Hydrolytic decomposition of protein and release of amines and amino acids by heterotrophs (bacteria like Bacillus, Pseudomonas) in absence of O2.
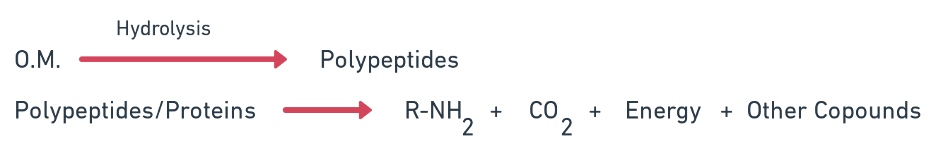
- The initial step in the decomposition of organic matter by the enzymatic digestion of proteins into amino acids like, peptones and amino acid.
- Under aerobic proteolysis the major end products are CO2 (NH4)2 SO4 and H2O. Under anaerobic conditions the end products are ammonia, amides, CO2 and H2S.
- The organic compounds and proteins are mainly decomposed by various species: Pseudomonas, Bacilli, Clostridium, Serrotia, Micrococcus.
- Generally in the neutral and sodic soils, bacteria are active and in acidic soils fungi are active.
Conversion of urea
- Urea is a product of ammonization. The hydrolysis of urea by the action of increases enzyme is effected by Bacilli micrococcus, Pseudomonas, Clostridium, Aeromobactor and Coryne bactor.
- CO (NH2) + H+ + 2H2O → 2NH4+ + HCO3-
- NH4+ → NH3 + H+
- 2NH3 + H2CO3 → (NH4)2 CO3 → NH4+ + CO32-
- The optimum H2O holding capacity for these reactions is 50% – 75% and optimum temperature is 30 – 50°C.
- The NH4+ can be utilized by microorganisms and root of higher plants. Some of the released NH3 is fixed by clay especially illite.
- A major portion is oxidized to nitrate form. In the second reaction NO2- is further oxidized to NO3- by nitrobactor.
- 2 NO2- + O2 → 2NO3-
N factor
- N factor is the No. of units of inorganic nitrogen immobilized for each 100 units of materials undergoing decomposition.
- The average values for the nitrogen factor vary from 0.1 or < to 1.3.
Ammonification
“Amines and Amino acids produced during aminisation of organic N are decomposed by other heterotrophs with release of NH4+ is termed Ammonifcation”.
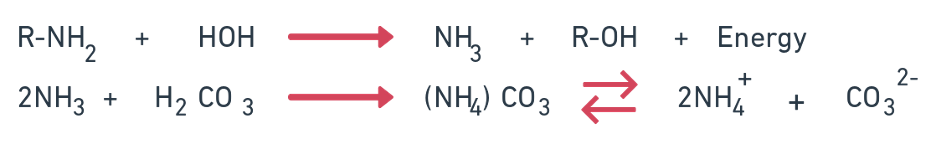
- NH3 so released are subjected to several fates:
- May be converted to Nitrites and Nitrates by nitrification.
- May be absorbed directly by higher plants.
- May be utilized by heterotrophs organisms in the further decomposition of O.M.
- May be fixed in a biologically unavailable form (in subsoil about 40-50% and in top soils 6 %) in the lattice of certain expanding type of clay-minerals like montmorillonite, illite and vermiculite.
Nitrification
- Nitrification is the process of biological oxidation by which the NH4+ form of N converts to nitrate form of N by
nitrobacteria(Nitrosomonas + Nitrobacter). There are two steps. - NH4+ is converted first to NO2- and then to NO3-.
- NH4+ →
Nitrosomonas→ NO2- (Nitrite) - NO2- →
Nitrobacter→ NO3- (Nitrate) - Optimum temperature for nitrifying bacteria is
30-35 °Coptimum pH is6.5-7.5. - Nitrosomonas are obligate autotropic bacteria that obtain their energy from the oxidations of N and their C from CO2
- Others autotrophic bacteria Nitrosolobus, Nitrospira and Nitroso vibrio and to some extend heterotrophic bacteria also can oxidize NH4+ and other N reduces, compounds to NO3-.
- When NH4+ - Fertilizers are added to soil, three important inferences are drawn by these nitrification equations:
- Reactions require molecular oxygen means nitrification takes place most readily in well aerated soil.
- Reactions release H+ which results in acidification of soil.
- Microbial activity hence their rapidity and extent of transformation will be greatly influenced by soil environmental conditions such as moisture supply, temp etc.
Losses of Nitrogen
- Leaching loss/drainage loss of NO3-
- Runoff loss like of NH3
- Gaseous losses
- The primary pathways of gaseous N losses are:
- Denitrification
- NH3 Volatilization
- Chemical Decomposition of NO2-
Volatilization/non-biological loss of NH4+
- NH4+OH- + H2O → NH3 (Loss) + 2H2O
- When PH > 8, nitrogen is lost in the form of NH3 in
alkaline medium. - Volatilization increase in poor drainage e.g.
rice field. - About 60 % of nitrogen loss in India is due to volatilization; hence in alkali soil the N application is raised at least by 25%.
- Gaseous losses of nitrogen (ammonia) are carried out by both volatilization and denitrification.
Denitrification
- Formation and loss of gaseous form of N by biological reduction of NO3- and NO2- are known as Denitrification.
- Denitrifying bacteria:
PseudomonasandBacillus. - Denitrification and volatilization occurs rapid at higher pH.
- Oxidation of NH3 to NO3- and NO2- and oxidation of carbon compounds run simultaneously as long as supply of elemental O2 is present. In the absence of elemental O2, NH4+ oxidation ceases.
- Certain organisms like Pseudomonas denitrificans (gram negative), Achromobactor, Bacillus, Micrococcus, are capable of using oxygen derived from nitrite and nitrate or both in place of elemental oxygen. Removal of oxygen from NO3- and NO2- reduces the substances chemically. The products of the reductions are mostly gaseous form of nitrogen including nitrous oxide (N2O), nitric oxide (NO) and elemental Nitrogen (N2).
- The most probable bio chemical pathway is
- NO3 (Nitrate) → NO2 (Nitrite) → NO (Nitric Oxide) → N2O (Nitrou Oxide) → N2 (Nitrogen)
- Waterlogging induces denitrification. Denitrification losses can be reduced by addition of phosphate residues, by providing adequate drainage and with availability of excess accumulation of active nitrogen compounds in the soil.
- After ratoon crop wheat yield is more due to availability of phosphatic fertilizer which is left as residue in the ratoon sugarcane and organic matter content is also high due to sugarcane ratoon.
Leaching loss
- Leaching or drainage loss mostly of
nitratefertilizer. - Mostly occurred in humid area.
Nitrogen fixation
- The conversation of elemental nitrogen to organic forms readily use able in biological process.
- Vermicullite and illite are capable of fixing NH4+ by a replacement of K+ or Na+ for interlayer cations in the expended lattice of clay minerals. The radius of NH4+ ion 0.143° A. K+ ion 0.133°A. Because of NH4 and K have more or less same charge, they easily replace each other in the exchange sites.
Organic fixation
- If the soil has more organic matter the NH4+ will lockup as a complex. There complex are called Chelates. It release the nutrients only after its molecular break down.
- The facultative aerobic bacteria tiles pseudomonas, bacillus, porococcus are responsible for denitrification. It also depends upon the texture of soil.
- In heavy clay soils loss is upto 50% of added fertilizer.
Elemental N loss
- It is due to chemical reduction.
- If chemical fertilizer containing amide (or) NH4 form of N, it may be oxidized to elemental N and lost.
- 2NHO2 + CO(NH2)2 → CO2 + 3H2O +2 NH2
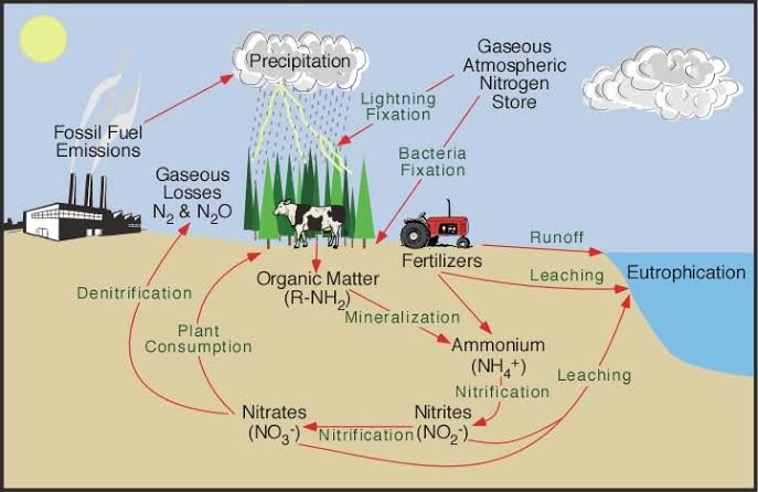
Nitrogen Cycle
- The cycling of N in the soil – plant – atmosphere system involves many transformations of N between inorganic and organic forms.
- The N cycle can be divided into
- N inputs or gains
- N outputs of losses
- N cycling with in the soil
- N in plant and animal residues and N derived from the atmosphere through electrical, combustion, biological and industrial process is added to the soil.
- N in the residues is mobilized as NH4 by soil organisms as an end product of residue decomposition, plant roots absorb a portion of the NH4.
- Much of the NH4 is converted to NO3 by nitrifying bacteria in a process called nitrifications.
- NO3 is taken up by the plant roots and is used to produce the protein in crops that are eaten by humans or fed to live stocks.
- Some NO3 is lost to ground H2O or drainage systems as a result of downward movement through the soil in percolating H2O.
- Some NO3 is consorted by denitrifying bacteria in to N2 and N2O that escape into the atmosphere, completely the cycle.
Important microorganisms in BNF
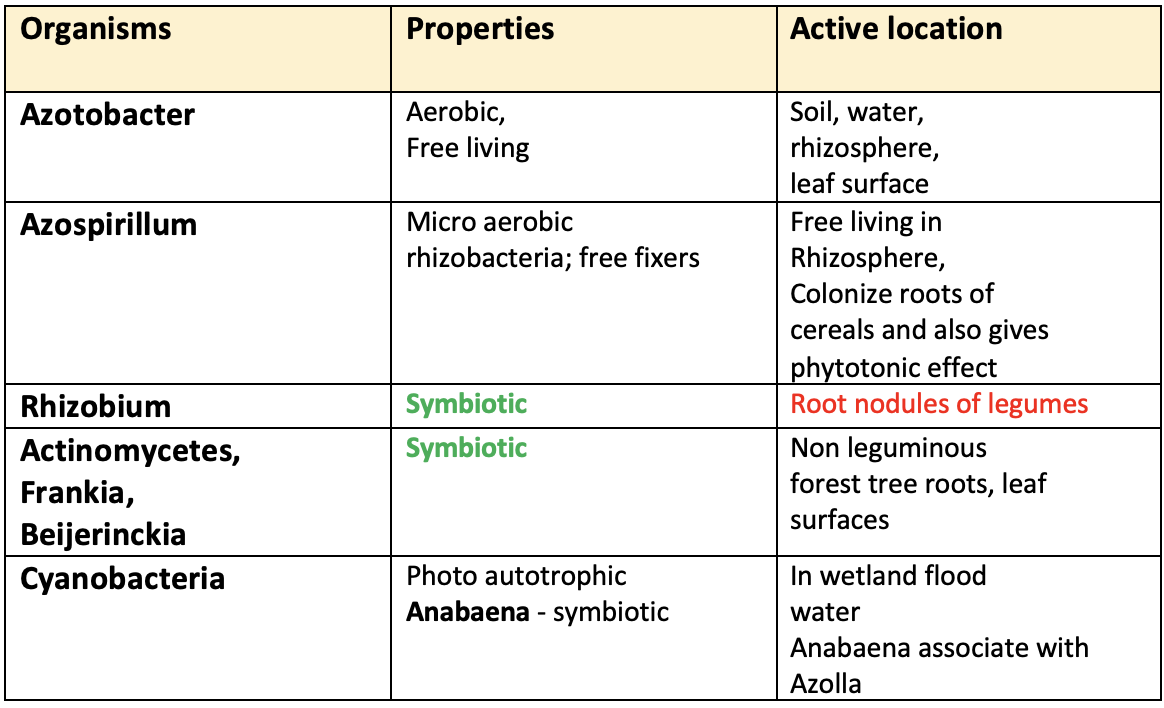
A. Symbiotic N fixation
- The symbiosis is the mutually beneficial relationship between host plant and bacteria.
- The location of association is in root or stem nodules, inside cavities, or by colonizing and penetrating plant tissue.
- The atmospheric nitrogen is fixed in to ammonia by an enzyme called
nitogenase.
1. Legume (nodule forming)
- Legumes and bacteria of the genera Rhizobium and Bradyrhizobium provide the major biological source of fixed N (40-60%) in agricultural soils. These organisms infect the root hairs and the cortical cells, ultimately inducing the formation root nodules that serve as the site of N fixation. The host plant supplies the bacteria with carbohydrates for energy and the bacteria reciprocate by supplying the plant with fixed N compound.
- Effective nodules cluster on primary roots and have pink to red centers. The red colour of the nodule is attributed to the occurrence of
leghemoglobin. - The quantity of N fixed by properly nodulated legume averages about 75% of the total N used for the plant growth. The amount of BNF varies with Rhizobium strain, host plant, and environment.
- Yield of non-legume crops often increase when they are grown following legumes (e.g. maize after soybean).
- Rhizobium is an aerobic and heterotrophic bacterium.
- It symbiotically fixes atmospheric nitrogen with the presence of leghaemoglobin in nodules of legume roots.
Rajmais a Pulse crop still it does not fix N from atmosphere.
👉🏻 Common bio strains of Rhizobium used commercially
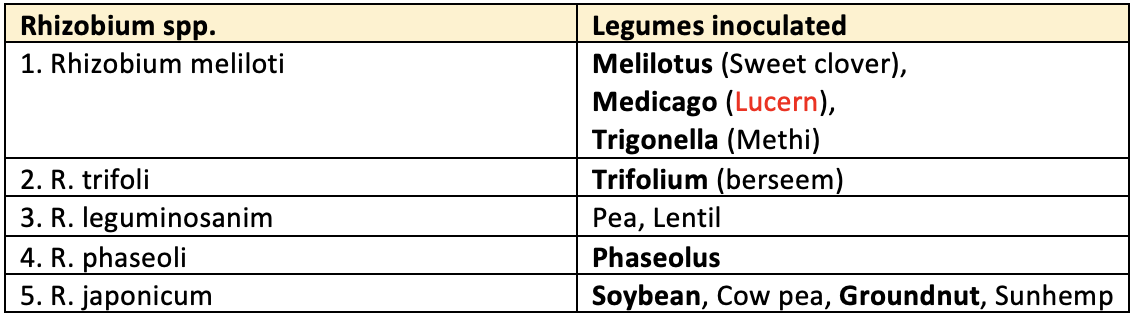
2. Non-legume (nodule forming)
- Roots of many species of angiosperm trees like
casuarinain forests and wetlands form distinct nodules when infected withActinomycetesof the genus Frankia. - Actinomycetes is bacteria not fungi.
3. Non-legume (non-nodule forming)
- Azospirillum, Azotobacter, and Azorhizobium, dominant N fixers in cereals like rice, wheat, corn, sorghum, millets can grow on root surfaces and penetrate root tissues. The organisms use carbohydrates of root exudates as source of energy. They also induce crop growth by hormonal action. The organism Beijerinckia fixes N on leaf surfaces of tropical plants.
- The Anabaena Blue Green Algae (Cyanobacteria) inhabit cavities in the leaves of the floating water fern Azolla pinnata (symbiosis) and fix quantities of N comparable to those of the better Rhizobium-legume complex. It could fix about 30-105 kg N per season taking care of 75% N requirement of rice.
- Azolla is widely used bio-fertilizer in
Rice. (Process called azofixation) NABARD 2021 - Azatobacter chrococcum: Free fixers commonly used for
Wheat,Rice,Cotton,Sugarcane. - Azospirillum: Associated with roots of grasses inside root symbiosis used for
Sorghum,Pearl Millet.
B. Non-symbiotic N fixation
- In wetland floodwater photoautotrophic Cyanobacteria independently do photosynthesis and fix N up to 20–30 kg N/ ha/ year. The excess ammonia is excreted in floodwater, which is beneficially absorbed by rice plants.
- Free-living heterotroph bacteria like
AzotobacterandBeijerinckiain aerobic upland soils andClostridiumin anaerobic wetland soils effectively fix N in pockets where O2 supply is limited. Fixation depends upon the pH, soil N level, and source of organic matter. Clostridium: Anaerobic Free Living Bacteria.
C. Industrial fixation of N
- Commercially produced N is the most important source of plant nutrient in agriculture.
- Industrial N fixation is by
Haber-Bosch process, in which H2 and N2 gases react to form NH3 under high temperature (1200 °C) and pressure (500 atm). - Anhydrous NH3 can directly be used as fertilizer or combined to other ions as solid forms.
D. N additions from atmosphere
- Ammonia escapes in to atmosphere from soils, manures, and industries because of volatilization. Organic N compounds remain in fine dust of air lifted from earth surface.
- Rainfall brings down to soil NH3, NO3-, NO2-, N2O, and organic N.
- About 10 to 20% of the NO3- in the rainfall is due to fixation of N2 by energy of lightning.
Functions of N
- In N sufficient plants, its concentration varies from 1 to 5 %. Cell cytoplasm and organelles contain N in combination with C, H, O, P and S.
- It’s an essential component of amino acids, proteins, nucleic acids, porphyrins, flavins, purines and pyrimidine nucleotides, nucleotides, enzymes, coenzymes and alkaloids.
- N containing
chlorophyllfixes atmospheric CO2 through photosynthesis. - Being a constituent of RNA and DNA, N is responsible for transfer of genetic code.
- Improves the quality of leafy vegetables and fodders.
- Improves the quality by increasing protein content.
- Feed Microorganisms in the soil. (Chemoautotrophs)
Deficiency of N
- Due to high mobility of N in plants it’s deficiency symptoms first appear in
older leaveswhich produces theyellowing or chlorosis. It appears first on the lower leaves, the upper leaves remain green, while under severe N deficiency lower leaves will turn brown and die. - Stunted growth is the manifestation. Ex. Buttoning in Cauliflower.
Uniform chlorosis of leaves including veins. The leaves become stiff and erect especially in cereals. Cereals show characteristic ‘V’ shaped yellowing at the tip of lower leaves.- Reduction in flowering and crop yields and lower protein content are associated with N deficiency.
- Cauliflower - Young leaves turn pale yellow and old leaves become orange.
- Coffee - Veins becomes yellow and new leaves are very small.
- Tomato - Stem become purple and hard. Flower buds become yellow and flower dropping rate also increases.
Excess of Nitrogen (Toxicity of nitrogen)
- Causes excess vegetative growth, dark green leaves, lodging, maturity is delayed with increases susceptibility to pest and disease.
- In cotton, weak fibre are resulted. In rice, lodging is common.
- Lengthening of crop duration and narrow leaf.
- Slender shoot, profuse vegetation, thick peel and skin will be rough and leathery in the case of citrus.
- Excess N in coffee plant, interferes the K uptake causing imbalance between N and K.
Explore More 🔭
🟢 https://www.youtube.com/watch?v=o1_D4FscMnU
References
- Tisdale, S.L., Nelson, W.L., Beaton, J.D. Havlin, J.L.1997. Soil fertility and Fertilizers. Fifth edition, Prentice hall of India Pvt. Ltd, New Delhi
- Singh, S.S.1995. Soil fertility and Nutrient Management. Kalyani Publishers, Ludhiana
- http://www.ncagr.gov/cyber/kidswrld/plant/nutrient.html
- Maliwal, G.L. and Somani, L.L. 2011. Soil Technology. Agrotech
- Soil Weight (i.e. furrow slice) = 2 x 106 kg/ha.
- On an average total nitrogen status of soil is 0.03 – 0.05% N means 1000 kg of N/ha.
- The cheap source of N is the crop residues in temperate region.
- In tropical soils, the total N (Available + Fixed) content is 0.03 - 0.1 %.
- 20-40% of total surface soil N is in the form of bound amino acids; 5-10% as combined hexose amines. Usually only 1-3% of the total amount of N present is mineralised during growing season.
- Rainfall is also source of N at 4.6 kg of N/ha is received ha-1 yr-1. It is converted to NO3- during lighting. Addition of organic matter and fertilizer is other major sources.
- Nitrogen is an essential constituent of protein and also other non-protein compounds of great physiological importance in plant metabolism.
- It is an integral …
Become Successful With AgriDots
Learn the essential skills for getting a seat in the Exam with
🦄 You are a pro member!
Only use this page if purchasing a gift or enterprise account
Plan
Rs
- Unlimited access to PRO courses
- Quizzes with hand-picked meme prizes
- Invite to private Discord chat
- Free Sticker emailed
Lifetime
Rs
1,499
once
- All PRO-tier benefits
- Single payment, lifetime access
- 4,200 bonus xp points
- Next Level
T-shirt shipped worldwide

Yo! You just found a 20% discount using 👉 EASTEREGG

High-quality fitted cotton shirt produced by Next Level Apparel