👯 Secondary Nutrients
Calcium, Magnesium, Sulphur
Calcium
- Calcium is absorbed by plants as Ca2+ and its concentration ranges from 0.2 to 1.0% and it is supplied through mass flow method.
Sources of soil calcium
- Earth crust contains about 3.64%. The important source of calcium is anorthite (CaAl2Si2O3).
- Generally arid region soils contain high amount of Ca regard less of texture, low rainfall and little leaching.
- In arid and semiarid regions:
- Calcite (CaCO3)
- Dolomite (CaMg(CO3)2)
- Gypsum (CaSO4, 2H2O)
- In humid regions, even the soils formed from limestone are frequently acidic on the surface layers because of the removal of Ca and other cations by leaching.
Calcium transformations in soils
- In acidic and humid region soils Ca occurs largely in exchange form and as primary minerals. In most of these soils Ca2+, Al3+ and H+ dominates the exchange complex.
- The forms of Ca are
- Solution Ca2+ / Mg2+
- Exchangeable Ca/Mg
- Mineral Ca/Mg
- The activity of solution Ca is decreased by leaching or plant removal. If solution Ca is increased, the equilibrium shifts in the opposite direction with subsequent desorption of some of the Ca2+ by the exchange complex.
- The rate of solution Ca is less complex than of K+.
- It may be
- Lost in drainage H2O
- Absorbed by organisms
- Adsorbed on the CEC
- Re-pricipitated as secondary calcium compound
Functions
- It is
immobilein plants and hence the deficiency is observed in younger leaves. - Essential for the formation of cell wall and calcium pectate in the middle lamella of the cell wall which regulates the entry of only those nutrients which are not toxic to plants. In seeds, calcium is present as calcium phytate.
- Promotes root (very essential for the meristematic activity) development and growth of plants, root elongation and cell division (highly required in Telophase for cell plate formation). AFO 2017
- Helps to translocate the sugar in the plants.
- It involves chromosome stability and that it is a constituent of chromosome structure.
- Affects translocation of CHO in plants.
- Encourages seed production.
- Activates enzyme phosphate and kinease.
- Accumulated protein during respiration by mitochondria and it increases their protein content. (Favors the assimilation of nitrogen into organic constituents)
- It binds DNA to protein molecules.
Deficiency
👉🏻 Soils seldom become calcium deficient, as long as soil pH is maintained towards neutral range. Deficiency of calcium is characterized by a reduction in meristematic tissue.
- Though calcium is highly mobile in soil, in the plant system it is the immobile nutrient and hence the deficiency symptoms manifest at the growing tips of shoots and youngest leaves.
- Bud leaf becomes chlorotic white with the base remaining green. About one-third chlorotic portion of the tip hooks downward and becomes brittle.
- No unfolding of new leaves in corn, whose tips are colorless and are covered with sticky gelatinous material which makes them adhere to one another.
- Chlorosis of young leaves followed by distortion of the growing points of the stem.
- In fruit trees, the death of growing points followed by
die back. - In apple, the discoloration of the fruit meat, the condition generally referred to as “
bitter pit”.

- In Brassica, severe loss of color in young leaves, terminal bud leaves are hooked, leaves below become cup shaped. Old leaves collapse due to terminal bud disintegration.
Blossom end rotin tomato is due to Ca deficiency
Magnesium
- Magnesium is absorbed as Mg2+ and the concentration in crop varies between 0.1 and 0.6%.
- It was taken by plant by Mass flow and diffusion.
Sources of soil Magnesium
- It constitutes 1.93% of earth crust
- Primarily minerals
- Biotite
- Dolomite
- Hornblende
- Olivine
- Serpentine
- Secondary minerals
- Chlorite
- Illite
- Montmorillonite
- In arid region substantial amount of Mg present as Epsomite (MgSO4.7H2O)
Forms of Magnisium in the soil
- It occurs predominately as exchange and solution Mg.
- Coarse texture soil exhibits the greatest potential for Mg deficiencies.
- Competition bet NH4+ and Mg2+ also lower the Mg2+ availability to crops.
Losses of Magnesium
- It is leached by soils and it depends on the Mg content, rate of weathering, intensity of weathering and uptake of plants.
Functions
- Mg is the only mineral constituent of
chlorophylllocated at its centre. AFO 2017/18 - Chlorophyll formation usually accounts for about 15 to 20 % of total Mg content of plants as Mg - porphyrin.
- Serves as a structural component of ribosomes. Mg activates the formation of polypeptide chains to form amino acids. About 70 % of Mg is associated with anions such as malate and citrate.
- Seeds contain Mg as salt of phytic acids. Mg is required for phosphate transfer from ATP (Phosphorylation) in carbohydrate metabolism.
- Several enzymes (eg: Ribulose carboxylose) require Mg2+ as Cofactor.
- It promotes uptake and translocation of phosphorus and movement of sugars within the plants.
Deficiency
- Mg2+ is a mobile element and is readily translocated from older to younger plant parts in the event of deficiency and hence deficiency symptoms are manifested in the older leaves.

Interveinal chlorosisof the leaf in which only the leave veins remain green.- Stiff brittle, twisted leaves, wrinkled and distortion of leaves.
- Cotton – lower leaves may develop a reddish purple finally necrotic (Redding of leaves).
- In brassica, Chlorosis with interveinal mottling uniformly distributed in older leaves while the other vascular tissues remain green. This condition is called “
Puckering”. Grass tetany: Cattle consuming forages with low Mg may suffer from “Hypomagnesemia” (low level of blood Mg) commonly known as Grass tetany. This happens due to high levels of NH4+ - N and K application.- Sand Drown in Tobacco.

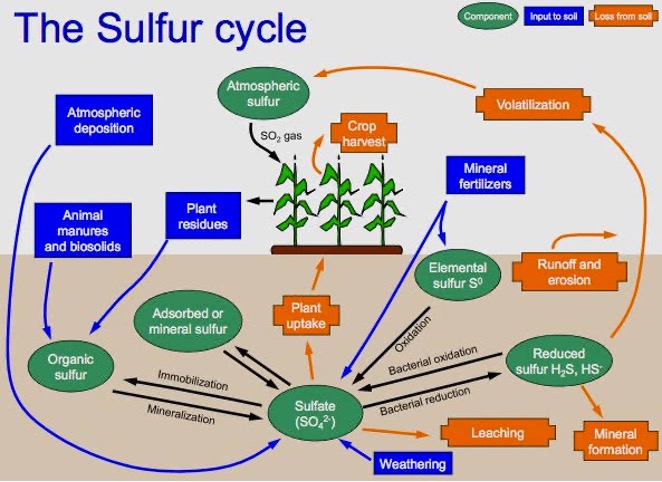
Sulphur
- Sulphur is absorbed by plant roots as SO42- ions. Concentration of ‘S’ in plants range between 0.1 and 0.4%.
Sources of Sulphur
- Amount has <0.05 ppm in the form of SO2
- Earth crust contains 0.06 to 0.10%
- Sulphur bearing minerals Gypsum - CaSO4.2H2O
- Epsomite - MgSO4.7H2
- Mirabilite - Na2SO4, 10 H2O
- Pyrite - FeS2
- Silicate minerals contains <0.01 % S
- Igneous rocks 0.02 to 0.07%
- Sedimentary rocks 0.02 to 0.22%
Forms of sulphur in soil
- Present both organic (90%) and Inorganic forms.
- The inorganic forms are:
- Solution SO42-
- Adsorbed SO42- 👉🏻 readily available fraction.
- Insoluble SO42-
- Reduced inorganic compounds
Factors affecting S oxidation in soils
- Microbial population in soil.
- Characteristics of the ‘S’ source
- Soil environmental condition.
- Soil micro flora Chemolithotropic S bacteria
Thiobacilliutilized energy from oxide of Inorganic S for the fixation of CO2 in Organic matter. - Soil temperature
- An increase in temperature increases the S oxidation rate in soil. Ideal temperature
25 – 40°C. - Soil moisture and aeration
- S oxidizing bacteria are mostly aerobic and their activity will decline if O2 is lacking due to H2O logging. Favorable moisture is field capacity moisture
- Soil pH: Optimum pH 4.0 or lower.
S transformation in soils
- Numerous transformations of S in soil occur from inorganic to organic forms due to the presence of heterotropic micro organic viz., Thiobacillus, Chlorabium, Desulfotomaculam and Desulfovibrio.
- When plant and animal residues are returned to the soil they are digested by microorganism releasing of the S as SO42-.
- Most of the S remains in organic form and becomes part of soil humus. The S supply to plants is largely depend on the SO42- released from the organic soil fractions and from the plant and animal residues.
S Mineralization and immobilization
- Mineralization of “S” is the conversion of organic S to inorganic SO42-
- Immobilisation is the conversion of SO42- to organic S.
- C: N ratio > 400 : 1 = Net Immobilization
- C: N ratio < 200 : 1 = Net Mineralization
- C: N ratio = 200 - 400 both immobilization and mineralization
Factors affecting S mineralization and Immobilization
- S content of organic matter: Mineralization of S depends on the S content of the decomposing material, smaller amounts of SO42- are liberated from low S containing residue.
- Source of mineralizable sulphur: Most of the available S removed by plants from the SO42- fraction of labile S in soil Organic matter.
- Soil Temperature: Mineralization of S is impeded @ 10°C increases with increasing temperature from 20°C to 40°C and decreases of temperature > 40°C.
- Soil moisture: Mineralization occurs in optimum moisture conditions.
- Soil pH: S released is directly proportional to pH up to pH 7.5. Above 7.5 mineralization increases more rapidly.
- Absence or presence of plants: Soils mineralization more S in the presence of growing plants than in their absence.
- Time and cultivation
- S Volatilization: Volatile S compounds are produced through microbial transformations under both aerobic and anaerobic conditions. The volatile compounds are dimethyl sulfide (CH3 SCH3) (CS2) Carbandisulphide and mercaptons (CH3SH). In low organic matter soils, S volatilization is negligible and increases with increasing organic matter content.
Practical aspects of S transformations
- Crops grown on coarse texture soils are generally more susceptible to S deficiency because these soils having low organic matter content and SO42- leaching.
- Leaching losses of SO42- can occur highly on coarse texture soils under high rainfall. Under such conditions, SO42- containing fertilizers maybe applied more frequently.
- Immobilization of added S can occur in soils having a high. C/S or N/S ratio. S mineralization is favored in soils with a low C/S or N/S ratio. S availability generally increases with organic matter content.
- Actual amount of S needed will depend on the balance between all soil additions of S by precipitation, air, irrigation H2O, crop residues fertilizers, Agriculture Chemicals and all losses through crop removal. Leaching and erosion.
Function
- It is required for the synthesis of the S containing amino acids
Cystein,CystineandMethionineand for protein synthesis. Animal protein is rich in ‘S’ than plant protein. - It activates certain proteolytic enzymes such as papainase and synthesis of papain. (Papaya)
- It is a constituent of certain vitamins viz.,
ThiamineandBiotin, coenzymes and Glutathione, Acetyl coenzyme A (precursor for fatty acid synthesis), ferredoxin. - It is present in the crops like Onion, Mustard, cabbage and Cauliflower as
polysulfides. (Pungency smell). - It increases
oil contentof crops like flax, soybean, groundnut etc. - Promote root growth and seed formation.
- Disulfide linkages (-S-S-) have been associated with the structure of proteins. Involved in forming and stabilizing the tertiary structure of enzymes and other proteins.
- Sulfhydryl (-SH) groups in plants are related to increased cold resistance.
- It is required for N fixation in legumes and is a part of nitrogenase enzyme system.
- Synthesis of chlorophyll.
Deficiency
👉🏻 As Sulphur is immobile in the plant, its deficiency is manifested on young leaves.
- Leaves small and veins are paler than interveinal portion, No dead spots; plants not lose the lower leaves as in N-deficiency. Vegetable leaves develop yellowish green colour and become thick and firm.
- Poor seed set in rapeseed.
- Tea: Tea yellows.
- In Brassica, the lamina is restricted and the leaves show cupping owing to the curling of leaves.
- In cabbage, there is a reddening and purpling of both upper and lower leaf surfaces.
- Reduced synthesis of proteins and oil.
Toxicity
- In some rice paddies, high in organic matter and low in active metallic elements such as iron, the free H2S may be released which is harmful to rice roots.
- This H2S injury to rice in paddies is referred to as “
Akiochi”.
References
- Tisdale, S.L., Nelson, W.L., Beaton, J.D., Havlin, J.L.1997.Soil fertility and Fertilizers. Fifth edition, Prentice hall of India Pvt. Ltd, New Delhi.
- Singh, S.S.1995.Soil fertility and Nutrient Management. Kalyani Publishers, Ludhiana.
- http://agropedialabs.iitk.ac.in/agrilore
- http://technology.infomine.com/enviromine/ard/microorganisms/roleof.htm
- http://www.agweb.com/article/the_secrets_of_sulfur/
Calcium
- Calcium is absorbed by plants as Ca2+ and its concentration ranges from 0.2 to 1.0% and it is supplied through mass flow method.
Sources of soil calcium
- Earth crust contains about 3.64%. The important source of calcium is anorthite (CaAl2Si2O3).
- Generally arid region soils contain high amount of Ca regard less of texture, low rainfall and little leaching.
- In arid and semiarid regions:
- Calcite (CaCO3)
- Dolomite (CaMg(CO3)2)
- Gypsum (CaSO4, 2H2O)
- In humid regions, even the soils formed from limestone are frequently acidic on the surface layers because of the removal of Ca and other cations by leaching.
Calcium transformations in soils
- In acidic and humid region soils Ca occurs largely in exchange form and as primary minerals. In most of these soils Ca2+, Al3+ and H+ dominates …
Become Successful With AgriDots
Learn the essential skills for getting a seat in the Exam with
🦄 You are a pro member!
Only use this page if purchasing a gift or enterprise account
Plan
Rs
- Unlimited access to PRO courses
- Quizzes with hand-picked meme prizes
- Invite to private Discord chat
- Free Sticker emailed
Lifetime
Rs
1,499
once
- All PRO-tier benefits
- Single payment, lifetime access
- 4,200 bonus xp points
- Next Level
T-shirt shipped worldwide

Yo! You just found a 20% discount using 👉 EASTEREGG

High-quality fitted cotton shirt produced by Next Level Apparel