🧉 Ion Adsorption
Ion Exchange, CEC, pH and Nutrient Availability
Adsorption of ions
- Ion adsorption and subsequent exchange are important processes that take place between soil colloidal particles (clays, organic matter, sesquioxides, and amorphous minerals) and various ions. Soil colloids serve very much as a modern bank. They are the sites within the soil where ions of essential plant nutrients are held and protected from excessive loss by leaching.
- Subsequently, the nutrients can be “withdrawn” from the colloidal “bank” sites and taken up by plant roots. In turn, these elements can be “deposited” or returned to the colloids through the addition of commercial fertilizers, lime, manures, and plant residues.
- The charges associated with soil particles attract ions (simple and complex) of opposite charge. In temperate region soils, negative charges generally predominate on the soil particles (colloids), hence adsorbed cations are present in larger quantities than anions.
- In more highly weathered soils (e.g. in tropics) where 1:1 type clays and Fe/Al oxides are the most dominant type of colloids, anion adsorption and exchange is relatively more prominent.
Ion exchange reactions
- The Ion Exchange phenomenon was first identified by Harry Stephen Thompson in England during 1850. When soil was leached (washed) with ammonium sulphate, calcium sulphate was detected in the leachate. The ammonium ion in the solution replaced calcium in the soil.

- The process by which ions are exchanged between solid and liquid phases and /or between solid phases if in close contact with each other is called ion exchange.
- The common exchangeable cations are Ca2+, Mg2+ H+, K+, NH4+ and Na+. The common anions are SO4 2-, Cl-, PO4 3- and NO3-
- The ion exchange in soil is due to the presence of residual positive and negative charges on the soil colloids. These residual charges result due to the process of
isomorphous substitutionandionisation of hydrogen and hydroxide functional groups. The negative charges attract positively charged ions and the positive charges attract negatively charged ions from soil solutions coming in contact with colloids. The ions thus attracted are reversible and are on equivalent proportions. - Exchange of cation is called
cation exchangeand exchange of anion is calledanion exchange. - The cation exchange phenomenon was first discovered by Thomasway (1850) Ion exchange is the second most important reaction is nature. The first one is photosynthesis by green plants.
- The capacity of the soil to hold cation is called cation exchange capacity. The unit is C mol (P+) / kg. cmol means centimol.
- Previously it was expressed as milli-equivalent/100 gm soil i.e., milli-equivalent.
- The capacity to hold anion is called Anion exchange capacity (AEC). The unit of expression is C mol (e-) / kg.
Mechanism of cation exchange
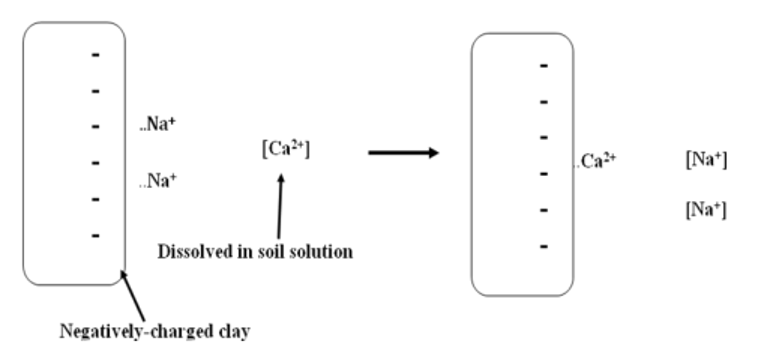
- Clay colloids have negative charges. Cations are attracted to the clay particles. These cations are held on the clay surfaces electro statistically.
- They are held by small particles of clay and organic matter. These small particles are called Micelle (Micro cell).
- Many positively charged ions or cations are attracted to micelles and formed ionic double layer, also called
Holmontz double layer.
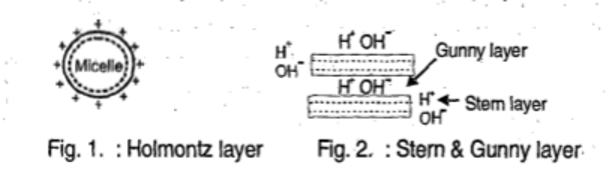
- A large no. of water molecules are carried by the adsorbed cations. Since most of them are definitely hydrated is called stem layer. Some silicate clays, in addition, hold numerous water molecules as well as cations packed between the plates (internal surface area) that make up the clay micelle is called gunny layer.
- The cations that can be replaced on exchange site by other cations are called exchangeable cations. They are weekly held and they are in direct contact with the soil solution. They can be exchanged fairly easily.
- Ions that are held very lightly with the colloid may be traped between layers of clay micelle. They do not pass to the soil solution very easily. They are called non exchangeable cations.
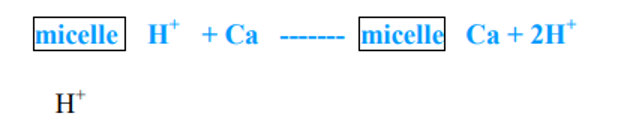
- When any cation is added to the soil such as Ca++, K+ or NH4+ through fertilizers and soil amendments they are exchanged with those ions held on the colloid.
- When calcium is added to an acid soil the following reaction takes place.
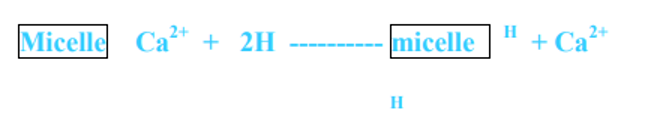
- Similarly when H+ is added to the soil solution through the decomposition of organic matter or through acidic materials Ca2+ is replaced from the exchange complex by H+.
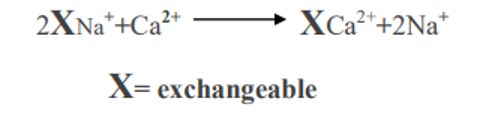
Cation exchange capacity (CEC)
- The sum total of the exchangeable cations that a soil can adsorb is called as cation exchange capacity. It is also defined as “the amount of cationic species bound at pH 7.0.
- Some authors consider pH 4.0 as the appropriate point.
Cation selectivity
Al 3+ > Ca 2+ > Mg 2+ > K = NH4+ > Na+
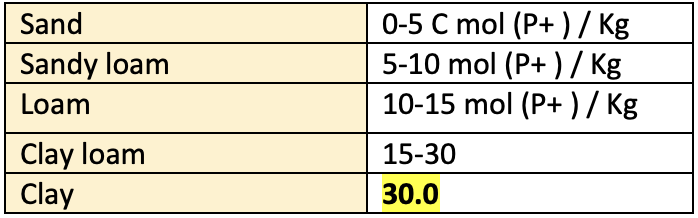
CEC of different textural classes
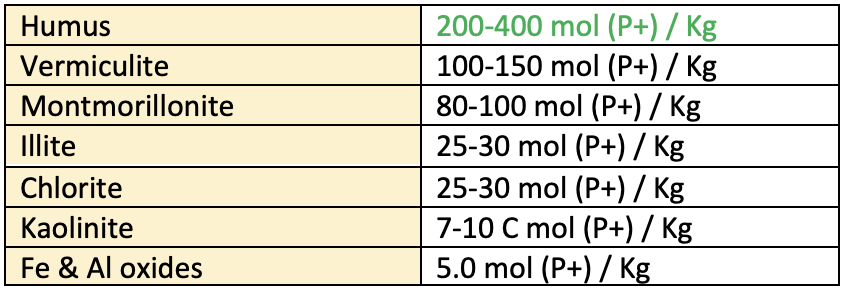
CEC of important clay minerals
Factors influencing CEC
- Soil Texture: CEC increases with fineness of the soil particles. This means increasing clay content will increase the CEC. As the particle size decreases the surface area increases per unit volume. This naturally increases the net charge and the CEC.
- Organic matter: In general CEC increases with increase in organic matter content. The pH dependent charges in the organic matter cause variation in CEC.
- Nature of clay: CEC of clay minerals vary soil dominated with montmorillonite and vermiculite have higher CEC than those dominated with kaolinite, chlorite or illite.
- Soil reaction: In general CEC increases with increase in soil pH. As the pH increases the pH dependent charge increases. In Humus most CEC is pH dependent.
Replacing power of ions
- Replacing power of ions increases with atomic weight.
- Divalent cations have **more **replacing power than monovalent ions.
- Hydrogen is an exemption. H ions are adsorbed more strongly than other monovalent or divalent ions. The replacing power of cations varies with the type of ion, size, degree of hydration, valence, concentration and the kind of clay mineral involved. As it is controlled by number of factors no single order of replacement can be given. All other factors being equal the replacing power of monovalent cations increases in the following order:
Li < Na < K < Rb < Cs < H
- and for divalent cations:
Mg < Ca < Sr < Ba
- In case of mixture of monovalent and divalent cations as they exist in normal soils the replacing power increases in the following order:
Na < K < NH4 < Mg < Ca < H
- This means Na is more easily replaced than K and K more easily than NH4.
- In general the power of replacement is
- Order of strength of Adsorption or ability to flocculate soil colloids in decreasing order:
Al3+ > H+ > Ca2+ > Mg2+ > K+ > Na+
- Maximum adsorption strength is exhibited by Al3+ and the least by Na+. Na+ results in a dispersed condition of soil colloids. Ba2+ flocculates solid colloids.
Base saturation***
- The percentage of CEC that is satisfied by the base forming cations is called base saturation percentage.

- Aluminum and hydrogen are considered as acid forming ions. Calcium, Magnesium, Potassium and Sodium are considered as base forming ions. Base forming substances are called as besoids and acid forming substances are called as acidoids.
- The percentage of sodium in the total CEC is called Exchangeable sodium percentage (ESP) [(Na/CEC) x 100]. These parameters are considered while deriving fertilizer prescription and amendments for problem soils. A knowledge of base saturation percentage is useful in many ways.
- It helps in determining the quantity of lime required to raise the pH of acid soils.
- It indicates the proportion of plant nutrients in CEC. It is an index of soil fertility.
- Degree of saturation of a particular cation in CEC indicates the ease with which the cation can be released for plant nutrition. For example if calcium saturation is more, Ca can be very easily replaced from the exchange complex.
- For a fertile soil it is considered that the base saturation percentage should be more than 80.
- For every one unit decrease in pH there is decrease of 15% in base saturation.
- Anion exchange replacement of one anion by another anion on the positively charged colloids is called anion exchange, positive charges are due to OH of iron and aluminium, 1:1 clays and allophone (a morphous clays). Anion exchange is pH dependent.
- Lower the pH greater is the anion exchange.
- Soils with Kaolinite dominant clay have higher anion exchange capacity than montmorillonite or illite.
- The relative order of anion exchange is:
OH- > H2PO4- > SO42- > NO3- > Cl-
Importance of anion exchange
- The phenomenon of anion exchange is important for the release of fixed P in the soil.
- In acid soils the phosphorus is fixed as insoluble Al-Phosphate. Liming the acid soils release fixed P. Here the OH ion replaces H2PO4- from Al(OH)2 H2PO4
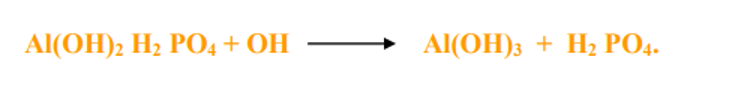
- Similarly the availability of other nutrients like NO3, SO4 and Cl- are influenced by anion exchange.
In soil Solution
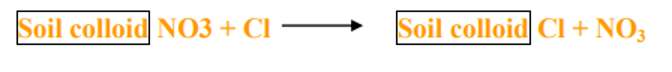
Significance of ion exchange
- Next to photosynthesis ion exchange is the most important reaction in the world.
- Plants take up their food material from the soil through ion exchange only.
- Plant roots which are in contact with the soil solution exchange the nutrients from soil solution for H+ ions present on the surfaces of root hairs.
Effect on soil fertility
- A soil is considered to be fertile when the base saturation percentage is
more than 80. - Each percent of humus contributes about 2 C mol /kg of CEC.
- Montmorillonite contributes about 1 C mole and Kaolinite contributes about 0.08 C mol/kg for every one percent.
Availability of applied nutrients
- When fertilizers are applied to supply plant nutrients elements like K, Ca, Mg and NH4 dissolve in soil solution. These nutrients in soil solution are exchanged for other cation like H+ present in the exchange complex. If there is no cation exchange the applied nutrients would be lost in drainage water.
- Similar is the case with anion radicals like PO4, NO3, SO4 etc. Soils with high CEC can adsorb higher amounts of nutrients.
- Hence, in clay soils we can apply larger quantities of fertilizers in a single dose.
- Sandy soils have very low CEC and in such soils fertilizers should be applied in splits.
Effect of adsorbed cations
- When the soil exchange complex has calcium the soil will have desire able physical properties. The activity of soil microorganisms, ammonification and nitrification processes also are determined by the cations of exchange complex.
Toxic ions
- When the exchange complex had adsorbed metals like Cadmium, Nickel and lead they are toxic to the crop plants.
Effect on soil pH
- Clays with H are acidic and with Na are alkaline.
- The acidic and alkaline nature of soil has its own effect on soil properties.
Soil pH
- Soil pH is a measure of the acidity or alkalinity in soils. In the pH scale, pH 7.0 is neutral. Below 7.0 is acidic and above 7.0 is basic or alkaline.
- Soil pH influences nutrient availability, soil physical condition and plant growth.
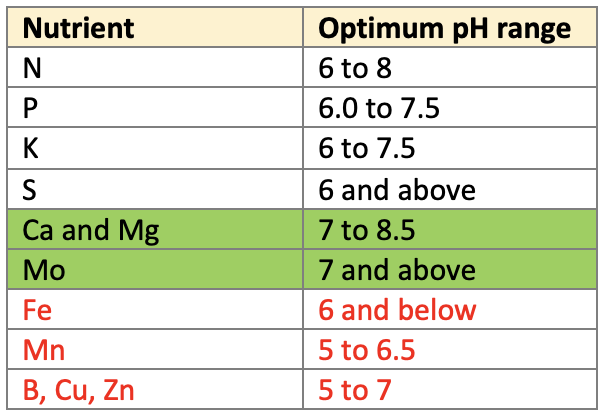
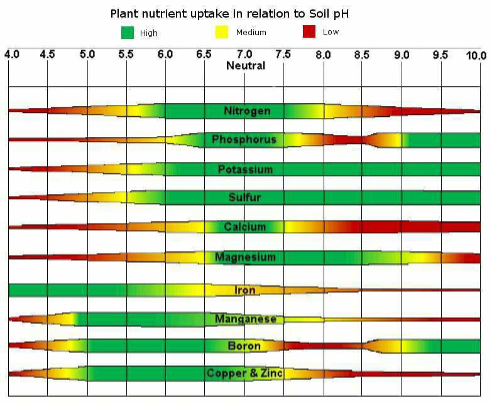
Nutrient Availability
- pH influences rate of nutrient release through its influence on the decomposition and solubility of materials.
For example
- The important source of nitrogen and sulphur is the organic matter.
- Decomposition of the organic matter is the slowest at pH below 6 and fastest between 6 to 8.
- Availability of
Fe, Al, Mn, Ca, Znare influenced by pH through its effect on the solubility of their nutrient compounds. - Availability of phosphorus is high within a pH range of
6.0 to 7.5due to higher solubility of phosphorus compounds. At low pH, phosphorus is precipitated. - Metallic cations like Fe, Mn, Cu, Zn precipitate at high pH, hence their availability is less in alkaline soils.
- At lower pH, H+ ions replace K and thus leaching of K occurs.
- At high pH, potassium compounds are converted into non-exchangeable form and thus the availability is reduced.
- Boron is leached out at low pH.
👉🏻 Optimum pH range for availability of different nutrients
- The deficiency of oxygen can inhibit absorptions of ions.
Adsorption of ions
- Ion adsorption and subsequent exchange are important processes that take place between soil colloidal particles (clays, organic matter, sesquioxides, and amorphous minerals) and various ions. Soil colloids serve very much as a modern bank. They are the sites within the soil where ions of essential plant nutrients are held and protected from excessive loss by leaching.
- Subsequently, the nutrients can be “withdrawn” from the colloidal “bank” sites and taken up by plant roots. In turn, these elements can be “deposited” or returned to the colloids through the addition of commercial fertilizers, lime, manures, and plant residues.
- The charges associated with soil particles attract ions (simple and complex) of opposite charge. In temperate …
Become Successful With AgriDots
Learn the essential skills for getting a seat in the Exam with
🦄 You are a pro member!
Only use this page if purchasing a gift or enterprise account
Plan
Rs
- Unlimited access to PRO courses
- Quizzes with hand-picked meme prizes
- Invite to private Discord chat
- Free Sticker emailed
Lifetime
Rs
1,499
once
- All PRO-tier benefits
- Single payment, lifetime access
- 4,200 bonus xp points
- Next Level
T-shirt shipped worldwide

Yo! You just found a 20% discount using 👉 EASTEREGG

High-quality fitted cotton shirt produced by Next Level Apparel