🏔 Minerals
Rocks, Classification of Soil Minerals
Rocks
- Rocks are the materials that form the essential part of the Earth’s solid crust.
- “Rocks are hard mass of mineral matter comprising one or more rock forming minerals”.
- Rocks are formed from the molten material known as magma.
- The study of rocks is called
Petrology(in Greek, petra means rock, logos means science). - Petrology deals with the description of rocks.
- Petrogenesis is the study of the origin of rocks.
Formation of rocks
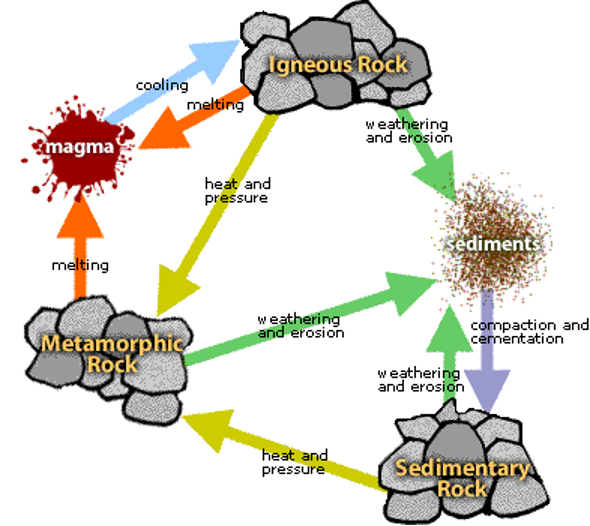
- Cooling and consolidation of molten magma within or on the surface of earth = Igneous or Primary Rocks
- Transportation and cementation of primary rocks = Sedimentary or Secondary Rocks
- Alteration of the existing primary and secondary rocks = Metamorphic Rocks
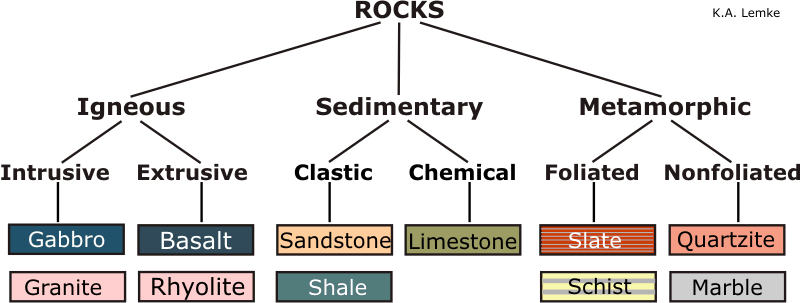
1. Igneous rocks (primary or massive rocks)
- These are first formed in the earth crust due to the solidification of molten magma. Based on the mode of formation, they are further classified as extrusive and intrusive rocks.
- Extrusive rocks or volcanic rocks
- The cooling of lava takes place on the surface of the earth fairly rapidly and the solidified material, known as volcanic rock and is glassy in structure.
- E.g. Andesite, Rhyolite and basalt.
- Basalt is prominent in Deccan trap area.
- Intrusive rocks or plutonic rocks
- Originally formed inside the earth by the solidification of the magma and pushed to the surface by various earth movements in pre-geological periods. Cooling inside the earth was a slow process. During cooling crystals developed and the rocks that formed were crystalline. When cooling was very slow, large crystals formed.
- E.g. Granite, Gabbro, syenite, diorite etc.
- Rocks formed in vertical cracks are called
dykesand in horizontal cracks are calledsills.
- Vesicular rocks: Molten magma cools on the surface. Steam of water is entrapped into rocks and forms vesicles.
2. Sedimentary rocks
- Such rocks are derived from igneous rocks hence
secondaryformations and are formed by the consolidation of fragmentary rock materials and the products of their decomposition deposited by water, glacier, wind and gravity (colluvial soils). - Example: Limestone, Dolomite, Sandstone, Shale, Conglomeration.

3. Metamorphic rocks
- These are formed from igneous and sedimentary rocks under the influence of heat, pressure, chemically active liquids and gases. Change may occur in mineral composition or texture or both.
- The changes due to
wateris called hydro metamorphosis and due topressureis called dynamo metamorphosis.

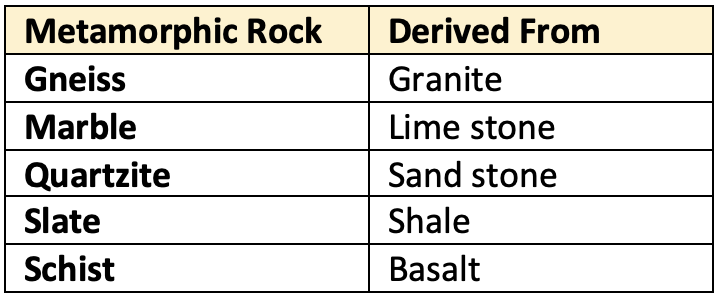
Based on the silica content, rocks are also classified as

Rock Minerals
- Minerals are naturally occurring solids with a definite chemical composition and crystal structure.
“Solid substances composed of atoms having an orderly and regular arrangement”.
- When molten magma solidifies, different elements present in them freely arrange in accordance with the attractive forces and geometric form.
- Silica tetrahedron is the fundamental building blocks for the formation of different minerals (SiO2).
- Different silicate minerals are ortho silicates, ino-silicates, phyllosilicates and tectosilicates.
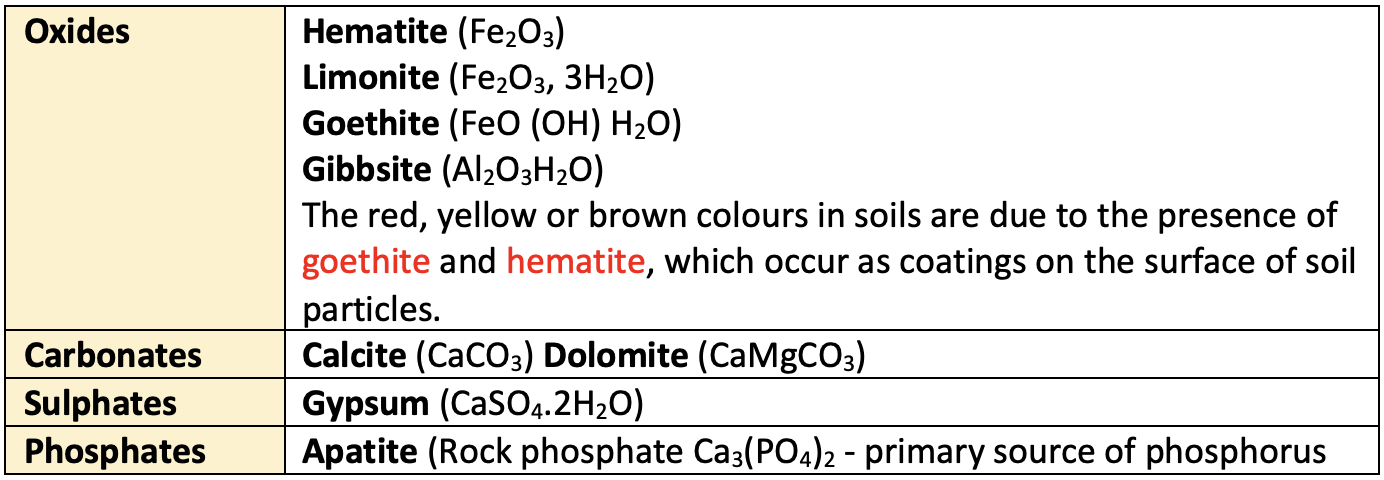
- There are nonsilicate minerals also. These are different oxides, carbonates, sulphates, phosphates etc.
Silicate minerals
Ortho/ Neosilicates
- The minerals in this group are composed of single tetrahedral linked together by Mg or Fe. To effect a break down, it is considered sufficient to sever the weaker Mg-O or Fe-O bonds.
- Non-withstanding the bond energy considerations susceptibility of the minerals in this group to breakdown by weathering appears to vary considerably from one mineral to another, e.g., zircon makes the mineral comparatively hard.
- On the other hand, the looser packing of oxygens in olivine makes the mineral weather faster.
Inosilicates
- The inosilicate group has in its structure single-chain (pyroxenes) and double chain (amphiboles) silica tetrahedral linked together by Ca, Mg, or Fe. Because of the presence of many weak spots provided by the Ca-O, Mg-O, or Fe-O bonds, these minerals tend to weather rapidly.
Phyllosilicates
- Linkages of silica tetrahedral and Alumina octahedral sheets by mutually shared oxygen atoms from the basis for the structure of this group.
- Some of the minerals, e.g., biotite and muscovite, are relatively susceptible to weathering, whereas others, like clay minerals, are resistant weathering products and further breakdown of clays is difficult. Disruption of interlayer ions, or through cleavage of Al-O bonds in tetrahedral and octahedral positions.
Tectosilicates
- The minerals are considered solid solution minerals with a framework of silica tetrahedral, in which the cavities are occupied by Na, Ca, and so on.
- The minerals in this group may also vary considerably in their resistance to weathering, e.g., leucite and plagioclase versus potash fertilizers.
- The relative degree of close packing of atoms in their structural frame work may be the reason for such variability in weathering.
- Increased substitution of Al and Si in tetrahedral of plagioclase mineral is also considered a factor that makes these minerals weaker than potash feldspars.
Non-silicate minerals
Primary minerals
- Primary mineral is the original component of rock, generally anhydrous and originally formed by cooling and solidification of molten mass
- These dominate the coarser fractions of soil viz. sands, gravel, stone.
- Examples:
Feldspar,Quartz,Mica, Hornblende, Orthoclase, Microcline, Plagioclase, Albite, Augite, Anothrite, Olivine.
Feldspars
- Feldspars are anhydrous aluminosilicates of K, Na and Ca and occasionally of other large cations such as Ba. The feldspar structure consists of tetrahedral which are attracted by sharing each oxygen atom between neighbouring tetrahedran. The tetrahedral contain mainly Silicons with sufficient Al substitution. It belongs to the group of minerals that are light in weight.
- Formula: K2O. Al2O3. 6SiO2
- Such mineral constitute about
48 %earth’s crust. - Feldspar weather easily and give rise to clay on hydrolysis.
Micas
- Micas constitute
10 %of earth’s crust. Two types:- Potash Mica: Potash mica is
white, clear and transparent and known asmuscovitemica. It is double silicate of K and alumina with a part of potash replaced by hydrogen: K(OH)2Al2Si3O10 - Magnesium Mica: It resembles muscovite mica but
blackin colour, also calledbiotic mica: K(Mg.Fe)3(OH)2AlSi3O10
- Potash Mica: Potash mica is
- White mica is more resistant to weathering than black mica.
- Micas are more resistant to weathering than feldspar and other silicates.
Olivine
- Ferro-magnesium silicate (Fe.Mg)2SiO4.
- Two hydrated forms of olivine are talc and serpentine, is a hydrated silicate of Mg.
Tourmaline
- It is boro-alumino-silicate.
- Non-ferromagnesium minerals
Quartz
- SiO2
- It is very densely packed and occurs in a high degree of purity. It is strongly resistant to weathering as the structure is densely packed, electrically neutral and free from any substitution.
- Chief constituent of sandy fraction
- Constitute of earth’s crust.
- Rocks containing free silica in abundance, which is not combined with bases, are called “acid rocks”.
- In granite (acid rock), quartz is present in a pure form as a prominent constituent.
- In basic rocks like basalt contain only a little, if any of free silica.
Resistance to Weathering
- Very slowly Weathered / Most resistant to weathering minerals
Quartz(💥 Most resistant)- Muscovite (More resistant)
- Moderate resistant/slowly weathered minerals
- Feldspar (orthoclase)
- Biotite
- Easily weathered / Least resistant minerals
- Augite
- Hornblende
- Olivine
Calcite(🤕 Least)
Secondary minerals
- Secondary minerals are formed from as a result of subsequent changes in rocks due to weathering or metamorphosis of primary minerals and hydrous in nature.
- These are most prominent in the fine materials especially in Clays.
- Examples:
Clay minerals(Kaolinitie, Montmorillonite, Illite etc.), Geothite, Haematite, Gibsite, Dolomite, Calcite, Gypsum. - Those minerals that are chief constituents of rocks are called as essential minerals (Feldspars, pyroxenes, micas etc) and those which are present in small quantities, whose presence or absence will not alter the properties of rocks are called accessory minerals (tourmaline, magnetite etc).
- Clay minerals in soils are formed from primary minerals due to weathering processes. These clay minerals are of size <0.002 mm and are considered to be the most reactive part of soil. Important soil properties like nutrient and water holding capacity are controlled by clay minerals. These minerals are layered silicates consisting of silica tetrahedron and aluminium octahedron.
- The most important silicate clay is known as
phyllosilcate. - The silicate clay unit consists of alternate sheets comprised of one type of sheet is dominated by silicon (Si) and other by Al/ Mg.
- Silica-dominated sheet is called
tetrahedraland Al/Mg sheet is calledoctahedralbecause of eight-sided building block. - Si4+ is surrounded by four oxygen ions and Al/Mg by six hydroxyl/oxygen ions.
Classification of silicate clays
A. On the basis of number and arrangement of tetrahedral and octahedral sheet, silicate clays are classified in to 3-different group:
- 1:1 type mineral: (one tetrahedral Si to one octahedral Al sheet): e.g. Kaolinite.
- 2:1 type mineral: (two Si sheet to one Al sheet). Means alumina is sandwiched between two tetrahedral silica sheets. Eg. Montmorillonite, Illite, Vermiculate and Micas.
- 2:1:1 or 2:2 type mineral: eg. Chlorites.
- These are basically ferro-magnesium silicates with some amount of aluminium present.
- It is having an extra layer of Mg-dominated alumina sheet, called Brucite (Mg (OH)2.
- But Mg2+ also dominates the alumina sheet of 2: 1 type minerals.
- Thus, crystal unit has two silica sheets and two magnesium dominated alumina sheet.
- Hence it is also called 2:2 type clay minerals.
- It is non- expanding in nature.
B. On the basis of expending and non-expending nature the silicate minerals are grouped in to following types: 1. Expanding type
- It includes montmorillonite (Smectite group) and vermiculite a. Montmorilionite:
- Montmorillonite is prominent in clay soils
- It is most common Smectite in soils where Mg+2 is substitute for Al+3 in alumina sheet (octahedral)
- It is loosened by “wander wall force”. b. Vermiculate:
- In this group the degree of swelling is considerably less than Smectite, so also called limited expansion clay mineral.
- But the CEC (cation exchange capacity) of vermiculites exceeds that of all other silicate clays due to very high negative charges. 2. Non-expanding type
- Illite: Found in clay soil
- Micas: (Muscovite, Biotite): These are found in sand and silt.
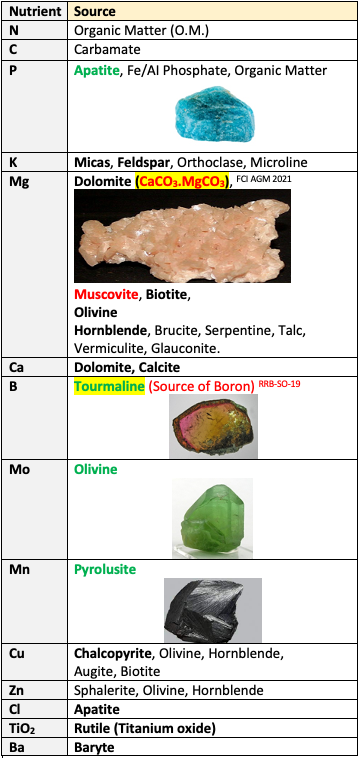
Sources of Plant nutrients
Rocks
- Rocks are the materials that form the essential part of the Earth’s solid crust.
- “Rocks are hard mass of mineral matter comprising one or more rock forming minerals”.
- Rocks are formed from the molten material known as magma.
- The study of rocks is called
Petrology(in Greek, petra means rock, logos means science). - Petrology deals with the description of rocks.
- Petrogenesis is the study of the origin of rocks.
Formation of rocks

- Cooling and consolidation of molten magma within or on the surface of earth = Igneous or Primary Rocks
- Transportation and cementation of primary rocks = Sedimentary or Secondary Rocks
- Alteration of the existing primary and secondary rocks = Metamorphic Rocks

1. Igneous rocks (primary or massive rocks)
- These are first formed in the earth crust due to …
Become Successful With AgriDots
Learn the essential skills for getting a seat in the Exam with
🦄 You are a pro member!
Only use this page if purchasing a gift or enterprise account
Plan
Rs
- Unlimited access to PRO courses
- Quizzes with hand-picked meme prizes
- Invite to private Discord chat
- Free Sticker emailed
Lifetime
Rs
1,499
once
- All PRO-tier benefits
- Single payment, lifetime access
- 4,200 bonus xp points
- Next Level
T-shirt shipped worldwide

Yo! You just found a 20% discount using 👉 EASTEREGG

High-quality fitted cotton shirt produced by Next Level Apparel