👾 Organic Matter
Fromation, Factors, Humus, Carbon Cylce
Soil Organic Matter
- Substances containing
carbonare organic matter. Soil organic matter consists of decomposing plant and animal residues. It also includes substances of organic origin either leaving or dead. - Soil organic matter plays an important role in deciding / maintaining soil physical conditions.
- It also influences soil chemical properties especially cation exchange capacity.
- Organic matters supply the
energy sourcesfor soil microorganisms. - Soil development is another aspect which is influenced by the soil organic matter.
- Plant tissue is the major source. Animals are considered as the secondary sources. They attack original plant tissues, contribute waste products and leave their own bodies after death.
- O.M. content of Indian soils is
low (0.5%)because of the high rate of decomposition under tropical and sub-tropical climate.
Soil organic matter fractions
- When soil is extracted with
alkali, the humic substances go into solution. The insoluble portion forms the non humic matter.- Humic matter (Soluble)
- Non humic matter (In-soluble)
Humic group
- This group makes up about
60-80%of the soil organic matter. - They are most complex. They are most resistant to microbial attack.
- Humic substances have aromatic ring type structures.
- These include polyphenols and poly quinones.
- These are formed by decomposition, synthesis and polymerization.
👉🏻 The humic substances are classified based on resistance to degradation and solubility in acids and alkalis into
- Fulvic acid: Lowest molecular weight and both acid and alkali soluble.
- Humic acid: Medium mol. wt. and alkali soluble but acid insoluble.
- Humin: High mol. wt. and both acid and alkali insoluble except under the most drastic conditions.
- Fulvic acid is most susceptible to microbial attack whereas humin is most resistant.
Non humic group
- This group makes upto
20-30%of the organic matter in soil. - These are less complex and less resistant to microbial attack as compared to humic substances.
- They are polysaccharides, polymers having sugar like structures and polyuronides.
- These include proteins, carbohydrates, lignins, fats, waxes, resins, tannins and some compounds of low molecular weight.
👉🏻 Factors affecting soil organic matter
- Climate
- Natural vegetation
- Texture
- Drainage
- Cropping and Tillage
- Crop rotations, residues and plant nutrients
1. Climate
- Temperature and rainfall exert a dominant influence on the amounts of N and organic matter found in soils.
- Temperature:
- The organic matter and N content of comparable soils tend to
increaseif one moves from warmer tocooler areas. - The decomposition of organic matter is accelerated in warm climates as compared to cooler climates.
- For
each 10°C declinein mean annual temperature, the total organic matter and N increases bytwo to three times.
- The organic matter and N content of comparable soils tend to
- Rainfall: There is an increase in organic matter with an
increasein rainfall. Under comparable conditions, the N and organic matterincreaseas the effective moisture becomes greater.
2. Natural Vegetation
- The total organic matter is higher in soils developed under
grasslandsthan those under forests.
3. Texture
Fine textured soilsare generally higher in organic matter than coarse textured soils because decomposition is slow in clay soils.
4. Drainage
Poorly drained soilsbecause of their high moisture content and relatively poor aeration are much higher in organic matter and N than well drained soils.
5. Cropping and Tillage
- The cropped lands have much low N and organic matter than comparable virgin soils.
Modern conservation tillagepractices helps to maintain high OM levels as compared to conventional tillage.
6. Rotations, residues and plant nutrients
- Crop rotations of cereals with legumes results in higher soil organic matter.
- Higher organic matter levels, preferably where a crop rotation is followed.
Decomposition of soil organic matter
- Different organic residues contain different organic compounds. There is great variation in the rate of decomposition of organic residues.
- Sugars, starches and simple proteins are very rapidly decomposed.
- On the other hand Fats, waxes and lignins are very slowly decomposed.
- Hemicellulose, celluloses and protein are intermediate.
- Even though the composition may vary the end products are more or less the same. The general reactions taking place during decomposition are
- Enzymatic oxidation of the bulk with the release of CO2, water, energy and heat
- Essential elements are released (N, P, S etc.) and immobilized by a series of reactions.
- Formation of compounds which are resistant to microbial action. Molecules very resistant to microbial action is formed either through modification of compounds or by microbial synthesis
👉🏻 Under aerobic conditions the products formed are CO2, NH4, NO3, H2PO4, SO4, H2O and essential plant nutrients like Ca, Mg, Fe, Cu, Zn etc.
👉🏻 Under anaerobic conditions CH4, organic acids like lactic, propionic, butyric, NH4, various amine residues (R-NH2) H2S, ethylene (CH2=CH2) and humic substances.
A. Decomposition of soluble substances
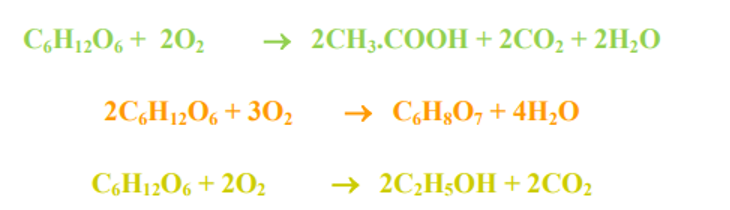
- When glucose is decomposed under aerobic conditions the reaction is as under:
Sugar + Oxygen → CO2 + H2O
Under partially oxidized conditions
Sugar + Oxygen → Aliphatic acids (Acetic, formic etc.) or Hydroxy acids (Citric, lactic etc.) or Alcohols (ethyl alcohol etc.)
Some of the reactions involved may be represented as under:
i) Ammonification
- The transformation of organic nitrogenous compounds (amino acids, amides, ammonium compounds, nitrates etc.) into
ammoniais called ammonification. - This process occurs as a result of hydrolytic and oxidative enzymatic reaction under aerobic conditions by heterotrophic microbes.
ii) Nitrification
- The process of conversion of ammonia to
nitrites(NO2) and then tonitrate(NO3 -) is known as nitrification. - It is an
aerobicprocess by autotrophic bacteria.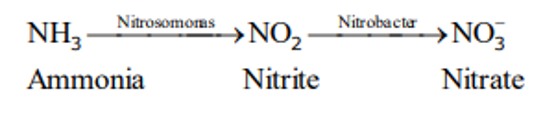
iii) Denitrification
- The process, which involves conversion of soil
nitrate into gaseous nitrogen or nitrous oxide, is called Denitrification. - Water logging and high pH will increase N loss by Denitrification.

👉🏻 Under anaerobic conditions
- C6H12O6 (Glucose) - Lactic acid, butyric acid, Ethyl alcohol are formed Protein and other N compounds are converted into elemental N.
B. Decomposition of Insoluble Substances
i) Breakdown of Protein
- During the course of decomposition of plant materials, the proteins are first hydrolyzed to a number of intermediate products.
- Aminization: The process of conversion of proteins to amino acids.
- Ammonification: The process of conversion of amino acids and amides to ammonia.
ii) Breakdown of cellulose
- The decomposition of the most abundant carbohydrates.
Cellulose → Cellobiose → Glucose → Organic acids → CO2 + H2O
Hydrolysis → Hydrolysis → Oxidation
- This reaction proceeds more slowly in acid soils than in neutral and alkaline soils. It is quite rapid in well aerated soils and comparatively slow in poorly aerated soils.
- Cellulose is not readily available for the use of bacteria but this is first acted upon by fungi arid changed into similar substances and made available for the use of bacteria.
- Wood is decomposed by
Actinomycetes.
iii) Breakdown of Hemicellulose
- Decompose faster than cellulose and are first hydrolyzed to their components sugars and uronic acids.
- Sugars are attacked by microbes and are converted to organic acids, alcohols, carbon dioxide and water.
- The uronic acids are broken down to pentose and CO2. The newly synthesized hemicelluloses thus form a part of the humus.
iv) Breakdown of Starch
- It is chemically a glucose polymer and is first hydrolyzed to maltose by the action of amylases.
- Maltose is next converted to glucose by maltase. The process is represented as under:
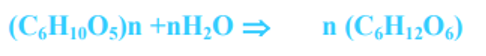
C. Decomposition of ether soluble substances
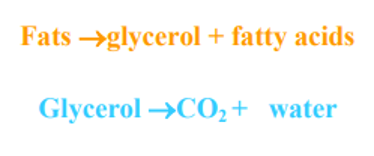
D. Decomposition of lignin:
- Lignin decomposes slowly, much slower than cellulose. Complete oxidation gives rise to CO2 and H2O.
Sulphur containing organic compounds:
- Converted to SO4-2 + H+ + energy by sulphur oxidizing bacteria.
P containing organic compounds:
- Various microorganisms mineralize phospholipids and other organic P compounds in the presence of phosphates enzymes H2PO4 and HPO4-2 depending on soil pH.
Factors affecting decomposition
- Temperature: Cold periods retard plant growth and organic matter decomposition. Warm summers may permit plant growth and humus accumulation. The soil organisms are most active at 24-35°C. O.M. of Indian soils is low because of the high rate of decomposition under tropical and subtropical climate. Except in a few localised areas in the hilly and high attitudes regions, the O.M. in most of the cultivated soils rarely exceeds 1% . O.M. content in Indian soil is generally
0.5%. - Soil moisture: When large quantities of O.M. are applied as manure in arid regions, the slender moisture in the soil may be largely used up for the decomposition of O.M. and the crop following may well suffer from lack of moisture. Extremes of both arid and anaerobic conditions reduce plant growth and microbial decomposition. Near or slightly wetter than field capacity moisture conditions are most favorable for both processes.
- Nutrients: Lack of nutrients particularly N slows decomposition.
- Soil pH: Most of the microbes grow best at pH 6 to 8, but are severely inhibited below pH 4.5 and above pH 8.5.
- Soil Texture: Soils higher in clays tend to retain larger amounts of humus.
- Other Factors: Toxic levels of elements (Al, Mn, B, Se, Cl), excessive soluble salts, shade and organic phytotoxins in plant materials.
Role of organic matter
- Organic Matters are sources of plant nutrients which are liberated in available forms during mineralisation. Humus can be considered to be a store-house of various nutrients essential to plant growth.
- During the slow microbial decomposition of the soil humus, there is a gradual release, with subsequent mineralization of C, N, S, P and other elements.
- It improves soil structure, its drainage and aeration, water holding capacity, buffer and exchange capacities; influences the solubility of minerals and serves as a source of energy for the development of micro-organisms. 95% N and 33% P of soil are obtained from O.M. It has a capacity to control soil temperature.
Humus
- Humus is a complex and rather resistant mixture of brown or dark brown amorphous and colloidal organic substance that results from microbial decomposition and synthesis and has chemical and physical properties of great significance to soils and plants.
- Humus is in dynamic condition.
- In humus,
40-45% ligninand30-33% proteinsand rests are fats, waxes and residual materials. Lignin and proteins constitute about 70-80% hence humus is also calledLigno-Protein complex. - In humus, the C: N ratio is
10 : 1. Humus is capable of adsorbing PO3- anions from soil solution but no other anions. - Mar: Raw humus, a type of forest humus layer of unincorporated organic material.
Humus Formation
👉🏻 The humus compounds have resulted from two general types of biochemical reactions: Decomposition and Synthesis.
- Decomposition:
- Chemicals in the plant residues are broken down by soil microbes including lignin.
- Other simpler organic compounds that result from the breakdown take part immediately in the second of the humus-forming processes, biochemical synthesis.
- These simpler chemicals are metabolized into new compounds in the body tissue of soil microbes.
- The new compounds are subject to further modification and synthesis as the microbial tissue is subsequently attacked by other soil microbes.
- Synthesis: Involve such breakdown products of lignin as the phenols and quinones.
- These monomers undergo polymerization by which polyphenols and polyquinones are formed.
- These high molecular weight compounds interact with N-containing amino compounds and forms a significant component of resistant humus.
- Colloidal clays encourage formation of these polymers.
- Generally two groups of compounds that collectively make up humus, the humic group and the nonhumic group.
Theories on humus formation
Lignin theory
- Proposed by Waksman (1936)
- According to this theory humic substances are formed due to the incomplete degradation of lignin.
Kononovas theory
- According to this theory humic substances are formed by cellulose decomposing mycobacteria earlier to lignin decomposition.
Polyphenol theory
- Flaig and Sochtig (1964)
- As per this theory the humic substances are formed by the condensation of phenolic materials.
- The polyphenols of lignin are oxidized to quinones.
- These quinones are condensed with low molecular weight microbial products to form humic molecules.
- The microbial products are amino acids, nucleic acid and phospholipids.
Properties of Humus
- The tiny colloidal particles are composed of C, H and O.
- The colloidal particles are
negatively charged(-OH, -COOH or phenolic groups), has very high surface area, higher CEC (150 – 300 cmol/kg) than that of silicate clays. Cation exchange reactions are similar to those occurring with silicate clays. - 4 - 5 times higher WHC
- Humus has a very favorable effect on aggregate formation and stability.
- Impart black colour to soils.
Carbon cycle
- Carbon is a common constituent of all organic matter (plant and animal residues).
- Carbon is continually being fixed into organic form by photosynthetic organisms under the influence of light and once bound, the carbon becomes unavailable for use in the generation of new plant life.
- Therefore, it is essential for the carbonaceous materials to be decomposed and returned to the atmosphere for the survival of the higher organisms.
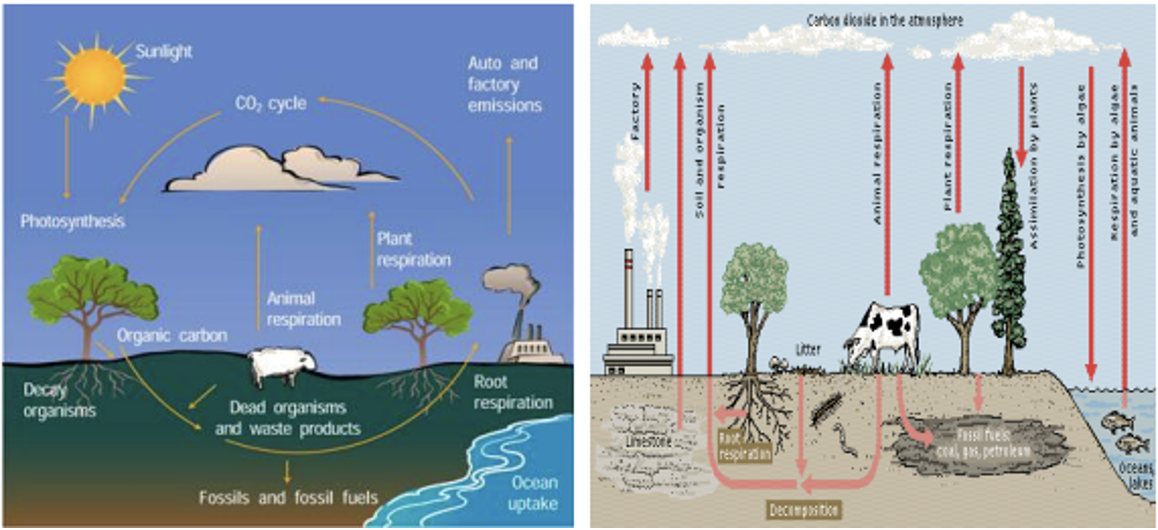
C: N ratio
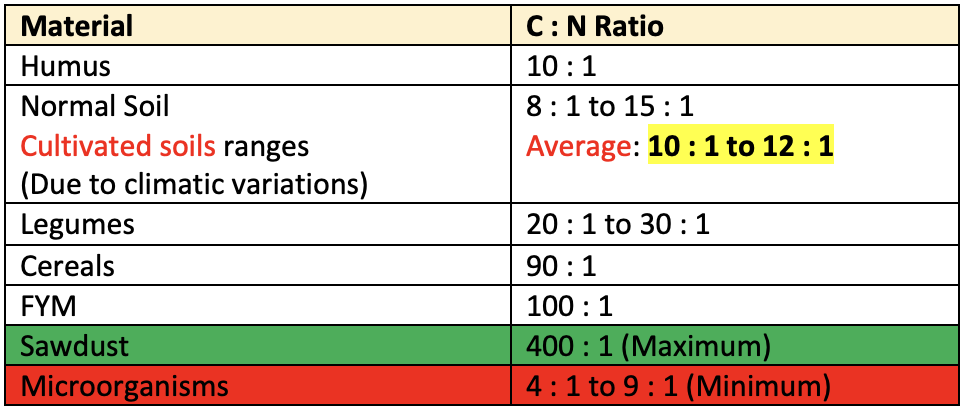
- The ratio of the weight of organic carbon to the weight of total nitrogen in a soil or organic material is known as C: N ratio.
- When fresh plant residues are added to the soil they are rich in carbon and poor in N.
- This results in wider C: N ratio (40:1) decomposition of the organic matter in the soil changes to humus resulting in a narrow C: N ratio (10:1).
- When materials high in carbon are added to the soil the microbial population increase due to the plentiful supply of food material.
- A lot of CO2 is released.
- Nitrogenous amendments increase CO2 evolution and greater loss of cellulose, hemicellulose and other plant polysaccharides.
Low N-contentorwide C: N ratioslows down decaying process. Therefore C: N ratio is used for predicting the rate of decomposition. It is important in controlling the available N, total O.M. The importance of C: N ratio in controlling the available nitrogen, total organic matter and rate of organic materials decomposition is recognized in developing appropriate soil management practices.- During this process the microorganisms utilize the soil N for their body build up and there is a temporary block of N.
- When the decomposition of fresh organic residues reaches to the stage where the C: N ratio is 20:1 there is an
increase in the availability of N. - The C: N ratio is lower in soils of arid regions than humid regions.
- C: N ratio is smaller in subsoil.
Significance of the C: N ratio
👉🏻 The C: N ratio in soil organic matter is important for two major reasons:
- The keen competition for available nitrogen results when organic residues of high C: N ratio are added to soils.
- Because this C: N (10:1) is relatively constant in soils, the maintenance of carbon and hence soil organic matter is dependent to no small degree on the level of soil nitrogen.
- So the C: N ratio obviously has practical implications on the availability of nitrogen in soils as well as in plants. As for example, large amount of fresh organic materials having wide C: N ratios (50:1) are incorporated into the soil under favorable soil conditions for decomposition. A rapid change will found. The heterotrophic microorganisms – bacteria, fungi and actinomycetes become active and increases their population with the production of large amounts of CO2. Under these conditions, nitrate nitrogen (NO3-N) disappears from the soil because of the urgent needs by the microorganisms, and for the time being, little or no nitrogen is available to plants. As the decomposition precedes, the C: N ratio on the organic materials decreases with the loss of carbon as CO2 and conservation of nitrogen.
- The older the plants the larger will be C : N ratio and the longer will be the period of Nitrate suppression.
- Leguminous tissues have a distinct advantage over non-legumes since it promotes a rapid organic turn over in soils. Legume stubbles decompose rapidly due to high N-content in the stubble.
- The practical significance of this relatively constant ratio is that a soil’s O.M. content cannot be increased without simultaneously increasing its Organic Nitrogen content and vice-versa.
58 g ‘C’ present in 100 g O.M.
1 g ‘C’ present in = 1.72 g O.M.
Therefore C: O.M. = 1 : 1.72
- 1.724 is called
Belmen Factor.
Muck and Peat soils

- Muck soils are having highly decomposed O.M. i.e. well mixed O.M. in the soil.
- Whereas in peat soils, mostly O.M. are partially decomposed and found under excessive moisture conditions.
- Peat soils are acidic, pH 3.9 and below, 10-40% O.M., suitable for paddy when water recedes.
Mineralization & Immobilization
- Mineralisation: The biological conversion of organic forms of C, N, P and S to inorganic or mineral forms as a result of microbial decomposition is called mineralization.
- Immobilization: The conversion of inorganic forms of C, N, P and S by the soil organism into organic forms is called Immobilization. Means reverse of mineralization.
Soil Organic Matter
- Substances containing
carbonare organic matter. Soil organic matter consists of decomposing plant and animal residues. It also includes substances of organic origin either leaving or dead. - Soil organic matter plays an important role in deciding / maintaining soil physical conditions.
- It also influences soil chemical properties especially cation exchange capacity.
- Organic matters supply the
energy sourcesfor soil microorganisms. - Soil development is another aspect which is influenced by the soil organic matter.
- Plant tissue is the major source. Animals are considered as the secondary sources. They attack original plant tissues, contribute waste products and leave their own bodies after death.
- O.M. content of Indian soils is
low (0.5%)because of the high rate of …
Become Successful With AgriDots
Learn the essential skills for getting a seat in the Exam with
🦄 You are a pro member!
Only use this page if purchasing a gift or enterprise account
Plan
- Unlimited access to PRO courses
- Quizzes with hand-picked meme prizes
- Invite to private Discord chat
- Free Sticker emailed
Lifetime
- All PRO-tier benefits
- Single payment, lifetime access
- 4,200 bonus xp points
- Next Level
T-shirt shipped worldwide

Yo! You just found a 20% discount using 👉 EASTEREGG

High-quality fitted cotton shirt produced by Next Level Apparel