💩 Soil Colloids
General Properties
- The colloidal state refers to a two-phase system in which one material in a very finely divided state is dispersed through second phase. The examples are: Solid in liquid (Dispersion of clay in water) and Liquid in gas (Fog or clouds in atmosphere).
- Colloidal particles are generally smaller than 1 micro meter (μm) in diameter. Since clay fraction of soil is less than 2 μm, therefore all clay is not strictly colloidal, but even the larger clay particles have colloidal properties.
General Properties of Soil Colloids
- Size
- The inorganic and organic colloids are extremely small size -
smaller than 2 micrometersin diameter. These particles cannot be seen using an ordinary light microscope but can be seen only with an electron microscope.
- Surface Area
- Because of their small size, all soil colloids have a larger external surface area per unit mass. The external surface area of 1 g of colloidal clay is 1000 times that of 1 g of coarse sand.
- Certain silicate clays have extensive internal surfaces occurring between plate like crystal units that make up each particle and often greatly exceed the external surface area.
- The total surface area of soil colloids ranges from 10 m2 /g for clays with only external surfaces to more than 800 m2 /g for clays with extensive internal surfaces. The colloid surface area in the upper 15 cm of a hectare of a clay soil could be as high as 700,000 km2 g-1.
3. Surface charges
- Both external and internal surfaces of soil colloids carry negative and/or positive charges.
- Most of the organic and inorganic soil colloids carry a negative charge.
- When an electric current is passed through a suspension of soil colloidal particles they migrate to anode, the positive electrode indicating that they carry a negative charge.
- The magnitude of the charge is known as
zeta potential. The presence and intensity of the particle charge influence the attraction and repulsion of the particles towards each other, there by influencing both physical and chemical properties. The sources of negative charge on clays comes from
Exposed Crystal Lattice
- (i) Ionization of hydroxyl groups
- (ii) Ionization of carboxyl and phenolic groups
- O2- and OH- groups are exposed at the broken edges and flat external surface as in Kaolinite: At pH > 7, the hydrogen of these hydroxyls dissociates slightly and the colloidal surface is left with a negative charge carried by oxygen.
- The loosely held H+ is readily exchangeable hence called pH-dependent charge of inorganic Colloids. This phenomenon apparently accounts for most of the CEC of 1 : 1 type colloidal clays and for organic colloids.
- Ionization of carboxyl (-COOH) or phenolic (C6H5OH) groups is the chief source of negative charges on humus micelles. With the increase in pH, extent of negative charge is increased, therefore is called pH-dependent negative charge or variable charge. As the soil pH increases, more OH- ions are available to force the reactions to the right; and negative charge increases.
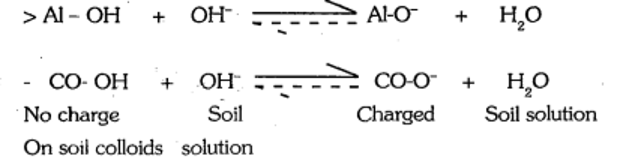
- Second possible point is that at high pH, complex aluminum hydroxy ions e.g. Al (OH)2+ is removed because these ions react with OH- to form insoluble Al (OH)3, thereby releasing negatively charged sites. But at low pH it blocks the negative sites and make them unavailable for cation exchange (the process of exchange of cations between solid and liquid phases).
- In the soils of temperate climates where 2:1 type clays are common, the permanent negative charges are usually dominant. In highly weathered soils of tropics where 1:1 type silicate clays, Iron and Aluminum oxides dominate and in soils high in O.M. the variable negative charges are more common.
Isomorphous substitution
- This is due to the substitution of a cation of higher valence with another cation of lower valence but similar size in the clay crystal structure. In clay crystals some ions fit exactly into mineral lattice sites because of their convenient size and charge.
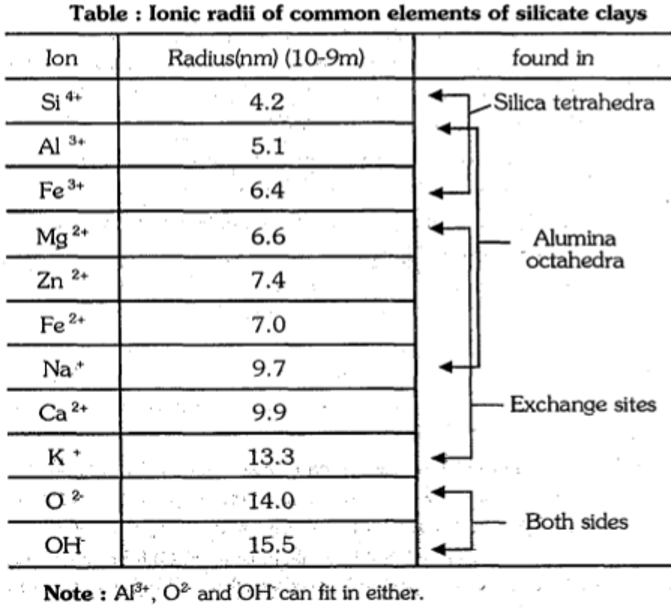
- Al3+ is slightly larger than Si4+ hence Al3+ can fit into the centre of tetrahedron in the place of Si4+ without changing the basic structure of crystal.
- In octahedron, Fe and Zn can fit into the position of Al or Mg. Positioning of Mg2+ in place of Al3+ or Al3+ in place of Si4+ leaves unsatisfied, negative charges from Oxygen anions in the sheets which account for overall negative charges from Oxygen anions in the sheets which account for overall negative charge of clay.
- Isomorphic substitution is of great significance in 2:1 type. The resultant negative charge is far in excess of that resulting from broken crystal edges of these minerals. Unlike the charge associated with the exposed crystal edges, those resulting from ionic substitution are not dependent on pH.
- The isomorphous substitution of any cation having lower charge results in an increase in positive charge. It is commonly, occurred in trioctahedral layers (Mg - dominated sheets) when Mg2+ is replaced by Fe3+/Al3+.
- Mg3(OH)6 → Mg2Al(OH)6+
- No substitution → Al3+ substituted for Mg2+
4. Adsorption of cations
- As soil colloids possess negative charge they attract and attach the ions of positive charge on the colloidal surfaces. They attract cations like H++, Al3+, Ca2+ and Mg2+. This gives rise to an ionic double layer.
- The Isomorphous substitution in the colloidal particle makes the external and internal layers of clay minerals negatively charged and these surfaces act as huge anions, which form the inner layer of the double layer.
- The outer layer is made up of a swarm of loosely held (adsorbed) cations attracted to the negatively charged surfaces.
5. Adsorption of water
- A large number of water molecules are associated with soil colloidal particles.
- Some water molecules are attracted to the adsorbed cations and the cation is said to be in hydrated state.
- Others water molecules are held in the internal surfaces of the colloidal clay particles. These water molecules play a critical role in determining both the physical and chemical properties of soil.
6. Cohesion
- Attractive force between similar molecules or materials
- Cohesion indicates the tendency of clay particles to stick together. This tendency is due to the attraction of clay particles for water molecules held between them.
- When colloidal substances are wetted, water first adheres to individual clay particles and then brings about cohesion between two or more adjacent colloidal particles.
7. Adhesion
- Attractive force between different molecules or materials
- Adhesion refers to the attraction of colloidal materials to the surface of any other body or substance with which it comes in contact.
8. Swelling and shrinkage
- Some soil clay colloids belonging to smectite group like Montmorillonite swell when wet and shrink when dry.
- After a prolonged dry spell, soils high in smectite clay (e.g. Black soil: Vertisols) often show crises-cross wide and deep cracks.
- These cracks first allow rain to penetrate rapidly. Later, because of swelling, the cracks will close and become impervious.
- But soils dominated by kaolinite, chlorite, or fine grained micas do not swell or shrink.
- Vermiculite is intermediate in its swelling and shrinking characteristics.
9. Dispersion and flocculation
- As long as the colloidal particles remain negatively charged, they repel each other and the suspension remains stable.
- If on any account they lose their charge, or if the magnitude of the charge is reduced, the particles coalesce, form flock or loose aggregates, and settle down.
- This phenomenon of coalescence and formation of flocks is known as flocculation.
- The reverse process of the breaking up of flocks into individual particles is known as de-flocculation or dispersion.
10. Brownian movement
- When a suspension of colloidal particles is examined under a microscope the particles seem to oscillate.
- The oscillation is due to the collision of colloidal particles or molecules with those of the liquid in which they are suspended. Soil colloidal particles with those of water in which they are suspended are always in a constant state of motion.
- The smaller the particle, the more rapid is its movement.
11. Non permeability
- Colloids, as opposed to crystalloids, are unable to pass through a semi-permeable membrane. Even though the colloidal particles are extremely small, they are bigger than molecules of crystalloid dissolved in water.
- The membrane allows the passage of water and of the dissolved substance through its pores, but retains the colloidal particles.
12. Acid Nature of Clay
- The clay being negatively charged is attracted by and shifts to the positive anode like acid radicles in an electrolytic medium.
- Therefore clay is sometimes referred to as clay - acid. In humid regions, H+ and Al3+ are predominant among cations and when it dissociates in the soil moisture, it imparts an acidic reaction to the soil solution.
- When H+ and Al3+ predominate in the clay complex as in humid regions, the clay is called Aluminum-Hydrogen clay or acid clay.
- In arid regions, Ca2+ and Mg2+ predominate in clay complex and the solution is more or less neutral and the clay is then called calcium clay.
- When percolation is considerably limited in arid regions, sodium salts accumulate on the soil surface and sodium becomes the predominant cation than calcium in the clay and is called sodium - calcium clay. A sodium calcium clay soil exhibits an alkaline reaction

- The colloidal state refers to a two-phase system in which one material in a very finely divided state is dispersed through second phase. The examples are: Solid in liquid (Dispersion of clay in water) and Liquid in gas (Fog or clouds in atmosphere).
- Colloidal particles are generally smaller than 1 micro meter (μm) in diameter. Since clay fraction of soil is less than 2 μm, therefore all clay is not strictly colloidal, but even the larger clay particles have colloidal properties.
General Properties of Soil Colloids
- Size
- The inorganic and organic colloids are extremely small size -
smaller than 2 micrometersin diameter. These particles cannot be seen using an ordinary light microscope but can be seen only with an electron microscope.
- Surface Area
- Because of their small size, all …
Become Successful With AgriDots
Learn the essential skills for getting a seat in the Exam with
🦄 You are a pro member!
Only use this page if purchasing a gift or enterprise account
Plan
Rs
- Unlimited access to PRO courses
- Quizzes with hand-picked meme prizes
- Invite to private Discord chat
- Free Sticker emailed
Lifetime
Rs
1,499
once
- All PRO-tier benefits
- Single payment, lifetime access
- 4,200 bonus xp points
- Next Level
T-shirt shipped worldwide

Yo! You just found a 20% discount using 👉 EASTEREGG

High-quality fitted cotton shirt produced by Next Level Apparel