🫐 Types of Colloids
Layer, Humus and other types
👉🏻 There are four major types of colloids present in soil
- Layer silicate clays
- Iron and aluminum oxide clays (sesquioxide clays)
- Allophane and associated amorphous clays
- Humus
👉🏻 Layer silicate clays, iron and aluminum oxide clays, allophane and associated amorphous clays are inorganic colloids while humus is an organic colloid.
Layer silicate clays - Phyllosilicates
- These important silicate clays are also known as
phyllosilicates(Phyllon - leaf) because of their leaf-like or plate like structure. - These are made up of two kinds of horizontal sheets. One dominated by silicon and other by aluminum and/or magnesium.
- Silica tetrahedron: The basic building block for the silica-dominated sheet is a unit composed of one silicon atom surrounded by
four oxygen atoms. It is called the silica tetrahedron because of its four-sided configuration. An interlocking array or a series of these silica tetrahedra tied together horizontally by shared oxygen anions gives a tetrahedral sheet. - Alumina octahedron: Aluminium and/or magnesium ions are the key cations surrounded by six oxygen atoms or hydroxyl group giving an eight sided building block termed octahedron. Numerous octahedra linked together horizontally comprise the octahedral sheet. An aluminum-dominated sheet is known as a di-octahedral sheet, whereas one dominated by magnesium is called a tri-octahedral sheet.
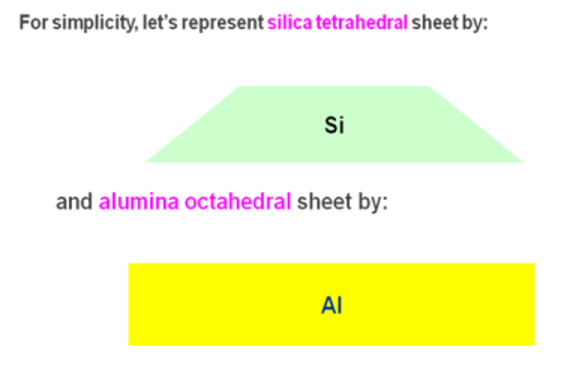
👉🏻 On the basis of the number and arrangement of tetrahedral (silica) and octahedral (alumina-magnesia) sheets contained in the crystal units or layers, silicate clays are classified into three different groups:
- 1 :1 Type clay minerals
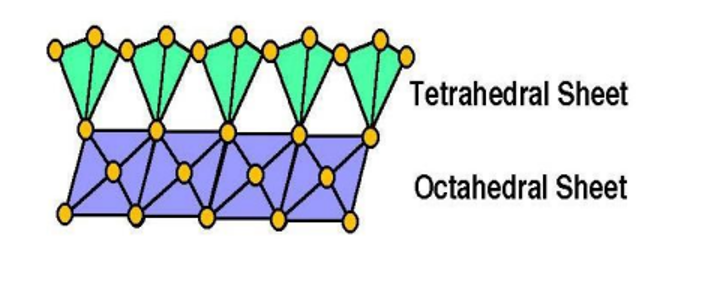
- 2:1 Type clay minerals
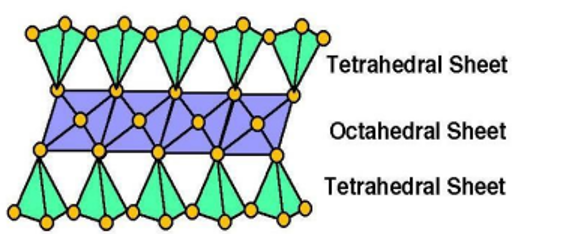
- Expanding type: Smectite group(Montmorillonte) and Vermiculite
- Non-expanding type: Mica group (illite)
- 2: 1: 1 (or) 2:2 Type clay minerals
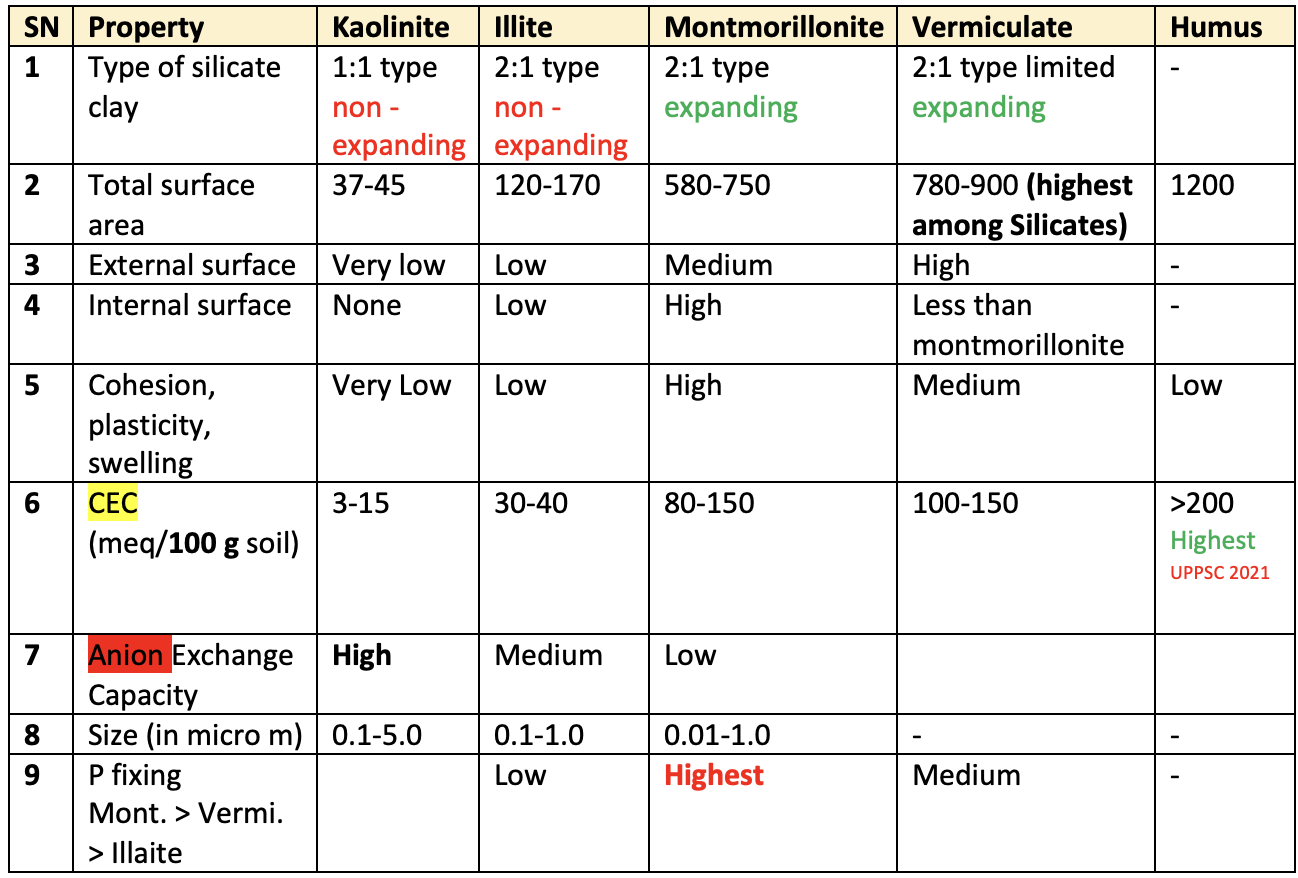
Basic properties of major silicate minerals and Humus
Iron and aluminum oxide clays (sesquioxide clays)
- Under the heavy rainfall conditions, soils are gradually leached out of bases and their place is taken up by H+ in the clay complex. When the H+ exceeds by a certain limit, silicate material in the soil is acted upon by them and free silicic acid is produced. It is soluble and is leached in drainage, and colloidal aluminium and Iron hydroxides are left behind. The silica content is reduced and the sesquioxides (Iron and aluminium oxides) become pre dominant in the residual clay. Such clay is called sesquioxide clay (sesquioxide → Fe2O3. Al2O3. TiOz).
- Sesquioxide clay does not possess the properties of plasticity and cohesion, has low base exchange capacity and low in fertility. The phosphorus in soil is tied up as Iron and alumiuium Phosphate.
- Sesquioxides (metal oxides) are mixtures of aluminum hydroxide, Al (OH)3, and iron oxide, Fe2O3, or iron hydroxide, Fe(OH)3. The Latin word sesqui means one and one-half times, meaning one and half times more oxygen than Al and Fe. These clays can grade from amorphous to crystalline.
Allophane and other amorphous minerals
- These silicate clays are mixtures of silica and alumina. They are amorphous in nature. Even mixture of other weathered oxides (iron oxide) may be a part of the mixture.
- Typically, these clays occur where large amount of weathered products existed.
- These clays are common in soils forming from volcanic ash (e.g., Allophane). These clays have high anion exchange capacity or even high cation exchange capacity.
- Almost all of their charge is from accessible hydroxyl ions (OH-), which can attract a positive ion or lose the H+ attached. These clays have a variable charge that depends on H+ in solution (the soil acidity).
Humus (Organic Colloid)
- Humus is amorphous, dark brown to black, nearly insoluble in water, but mostly soluble in dilute alkali (NaOH or KOH) solutions.
- It is a temporary intermediate product left after considerable decomposition of plant and animal remains. They are temporary intermediate because the organic substances remain continue to decompose slowly.
- The humus is often referred to as an organic colloid and consists of various chains and loops of linked carbon atoms. The humus colloids are not crystalline. They are composed basically of carbon, hydrogen, and oxygen rather than of silicon, aluminum, iron, oxygen, and hydroxyl groups.
- The organic colloidal particles vary in size, but they may be at least as small as the silicate clay particles. The negative charges of humus are associated with partially dissociated enolic (-OH), carboxyl (-COOH), and phenolic groups; these groups in turn are associated with central units of varying size and complexity.
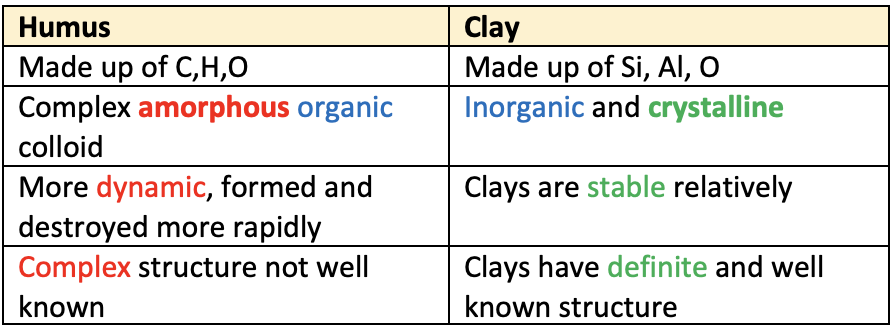
👉🏻 Difference between organic and inorganic colloids
👉🏻 There are four major types of colloids present in soil
- Layer silicate clays
- Iron and aluminum oxide clays (sesquioxide clays)
- Allophane and associated amorphous clays
- Humus
👉🏻 Layer silicate clays, iron and aluminum oxide clays, allophane and associated amorphous clays are inorganic colloids while humus is an organic colloid.
Layer silicate clays - Phyllosilicates
- These important silicate clays are also known as
phyllosilicates(Phyllon - leaf) because of their leaf-like or plate like structure. - These are made up of two kinds of horizontal sheets. One dominated by silicon and other by aluminum and/or magnesium.
- Silica tetrahedron: The basic building block for the silica-dominated sheet is a unit composed of one silicon atom surrounded by
four oxygen atoms. It is called the silica …
Become Successful With AgriDots
Learn the essential skills for getting a seat in the Exam with
🦄 You are a pro member!
Only use this page if purchasing a gift or enterprise account
Plan
- Unlimited access to PRO courses
- Quizzes with hand-picked meme prizes
- Invite to private Discord chat
- Free Sticker emailed
Lifetime
- All PRO-tier benefits
- Single payment, lifetime access
- 4,200 bonus xp points
- Next Level
T-shirt shipped worldwide

Yo! You just found a 20% discount using 👉 EASTEREGG

High-quality fitted cotton shirt produced by Next Level Apparel