🌩 Mutation Breeding
Procedure, For Oligogenic and Polygenic Traits
- When mutations are induced for crop improvement, the entire operation of the induction and isolation, etc. of mutants is called mutation breeding.
- Seeds, pollen grains or vegetative propagules (buds & cuttings) may be used for mutagenesis. In sexually propagated crops, seeds are the most commonly used plant part. In case of clonal crops buds or cuttings are used for mutagenesis. Chemical mutagens are best used with seeds.
- Whole plants are generally irradiated during the flowering stage so that it is equivalent to irradiation of pollen grains and egg cells. Treatment of whole plants requires special facilities i.e. a gamma garden.
Gamma garden
- It is an area subjected to gamma-irradiation. This area is enclosed by thick high walls. The purpose of y-garden is to irradiate whole plants during different stages of development and for varying durations. The source is in centre.
- First y-garden was built in Long island near New York (USA).
- The first y-garden in India was installed in Calcutta (Kolkatta) at Bose Research Institute in 1959.
- In 1960 2nd y-garden was built at IARI, New Delhi. 3rd y-garden was built at Bhabha Atomic Research Centre, Trombay.
- The source of y-garden was 6g of 60Co in form of small pellets. The strength of 60Co source was 200 curies.
- The y-garden at IARI & BARC and at most other places in the world have now been dismantled because of their high Cost.
Optimum dose of mutagen
👉🏻 Selection of the variety for mutagen treatment
- The variety selected for mutagenesis should be the best available in the crop.
- An optimum dose is the one which produces the maximum frequency of mutations and causes the minimum killing. A dose close of LD50 is the optimum.
- LD50 is the dose of mutagen which would kill 50% of the treated individuals.
- Treatment of seeds and vegetative propagules commonly produces chimeras.
- A chimera is an individual with one genotype in some of its parts and another genotype in the others.
Irradiation
- Exposure of a biological material to α-radiation like X-rays, y-rays etc. is known as irradiation. The primary effect of radiation is ionisation; the mechanism of ionisation differs to some extent from one radiation to other.
- Genetic effects of radiation i.e. effects on DNA means
- The change in a base i.e. deamination
- The loss of a base
- Breaking of hydrogen bonds in DNA
- Breaking in single and double strand of DNA
- Cross-linking of DNA strands
- Pyrimidine are more sensitive to radiation damage than purines
- Deamination and intrastrand dimerization of bases may lead to change base pairing producing changes in the base lead to sequence of DNA i.e. gene mutation.
Procedure for irradiation
👉🏻 The plant material may be treated in any of the following source.
- Seeds: Seeds are used after soaking to get greater frequency of induced mutations than air dried.
- Seedlings: At any stage of life cycle can be subjected to radiation but usually seedlings neither too young nor too old are irradiated due to their convenience in handling in pots transportation from nursery easily.
- Flowers: Meiotic cells have been found more sensitive than the mitotic cells and therefore plants are irradiated in the flowering stage in order to affect the developing gametes.
- Cuttings: In case of fruit tree when they are propagated by clones – the desirable cuttings are exposed to irradiation.
Mutation Breeding for oligogenic traits
👉🏻 The handling procedure described here is based on the selection for a recessive mutant allele of an oligogene.
- M1. Several hundred
seedsare treated with a mutagen and are space planted. In general, the number of treated seeds is so adjusted as to give rise to 500 fertile M1 plants at the harvest. Care should be taken to avoid outcrossing; this can be achieved either by planting the M1 population in isolation or by bagging the inflorescences of M1 plants or even the whole M1 plants. M1 plants will be chimeras for the mutations present in heterozygous state. About 20 to 25 seeds from each M1 spike are harvested separately to raise the M2 progeny rows. - M2. About 2,000 progeny rows are grown. Careful and regular observations are made on the M2 rows. But only distinct mutations are detected in M2 because the observations are based on single plants. All the plants in M2 rows suspected of containing new mutations are harvested separately to raise individual plant progenies in M3. If the mutant is distinct, it is selected for multiplication and testing. However, most of the mutations will be useless for crop improvement. Only 1 -3 per cent of M2 rows may be expected to have beneficial mutations. Alternatively, M2 may be grown as a bulk produced by compositing one or more, but equal number of, seeds from each M1 spike/fruit/branch. Individual plants are then selected in M2 and individual plant progenies are grown in M3.
- M3. Progeny rows from individual selected plants are grown in M3. Poor and inferior mutant rows are eliminated. If the mutant progenies are homogeneous, two or more M3 progenies containing the same mutation may be bulked. Mutant M3 rows are harvested in bulk for a preliminary yield trial in M4.
- M4. A preliminary yield trial is conducted with a suitable check, and promising mutant lines are selected for replicated multi-location trials.
- M5-M7. Replicated multilocation yield trials are conducted. The out-standing line may be released as a new variety. The low yielding mutant lines, however, should be retained for use in hybridization programmes.
Mutation breeding for polygenic traits
👉🏻 Mutagenesis does produce genetic variation in polygenic traits; this variation is usually as much as 50% of that generated in F2 generation, but sometimes it may be as much as or even greater than the latter.
- M1 and M2. M1 and M2 are grown in the same way as in the case of oligogenic traits. In M2, vigorous, fertile and normal looking plants that do not exhibit a mutant phenotype are selected and their seeds are harvested separately to raise individual plant progeny rows in M3.
- M3. Progeny rows from individual selected plants are grown. Careful observations are made on M3 rows for small deviations in phenotype from the parent variety. Inferior rows are discarded. Few rows may be homogeneous and would be harvested in bulk. Selection is done in M3 rows showing segregation; a majority of M3 rows would show segregation. Intensive and careful evaluation of a large number of M3 progeny rows allows identification of mutants with altered quantitative traits, e.g., partial or horizontal disease resistance. Such mutants occur in high frequencies that approach 1% or even high, so that their isolation becomes quite cost effective.
- M4. Bulked seed from homogeneous M3 rows may be planted in a preliminary yield trial with a suitable check; superior progenies are selected for replicated multilocation yield trials. Individual plant progenies from M 3 are critically observed. Progenies showing segregation may be subjected to selection only if they are promising. Superior homogeneous progenies are harvested in bulk for preliminary yield tests in M5.
- M5-M8. Preliminary yield trials and / or multi-location trials are conducted depending upon the stage when the progenies become homogeneous. Outstanding progenies may be released as new varieties.
Applications of Mutation Breeding
- Mutation breeding has been used for improving both oligogenic as well as polygenic characters.
- Mutagenesis has been used to improve morphological and physiological characters including yielding ability.
- Various applications of mutation breeding are:
- Induction of desirable mutant alleles which may not be available in the germplasm.
- It is useful in improving specific characteristics of a well-adapted high yielding variety.
- Mutagenesis has been successfully used to improve various quantitative characters including yield.
- F1 hybrids from intervarietal crosses may be treated with mutagens in order to increase genetic variability by inducing mutation and to facilitate recombination of linked genes.
- Irradiation of interspecific (distant) hybrids has been done to produce translocations.
✅ Advantages
- Mutation create inexhaustible variation.
- When no improvement is possible this method has to be adopted.
❌ Limitations
- Frequency of desirable mutations is very low about 0.1 percent. To detect the desirable one in M2 considerable time, labour & other resources are to be employed.
- To screen large population, efficient quick and unexpensive selection techniques are needed.
- Desirable mutations may be associated with undesirable side effects due to other mutations thus extending the mutation breeding programme.
- Detection of recessive mutations in polyploids and clones is difficult and larger doses of mutagen have to be applied and larger populations are to be grown.
🏆 Achievements
- Natural mutants:
- Rice:
- GFB 24 – arose as a mutant from Konamani variety Dee – Gee – Woo – Gen – Arose as a mutant from rice in China
- MTU 20 – arose as a mutant from MTU-3
- Sorghum: Co. 18 – arose as a mutant from Co. 2
- Cotton: DB 3-12 from G. heroaccum variety Western 1
- Rice:
- Induced mutants:
- Rice:
Jagannath- Gamma ray induced mutant from T.141 - Wheat:
Sarbati Sonora- Sonora-64 was gamma irradiated by M.S. Swaminathan and named them Sarbati-Sonora. - Cotton:
- Indore 2 Induced from Malwa upland 4
MLU 7gamma ray induced mutant from culture 1143 EEMLU 10gamma ray induced mutant from MLU 4
- Mustard: Primax whicte (1950)
- Sugarcane: Co.8152 gamma ray induced mutant from Co. 52
- Groundnut: NC 4
- Castor:
Aruna(NPH1) – Fast neutrons induced mutant from HC 6 - China has developed the largest number of mutant varieties followed by India, Russia & Japan.
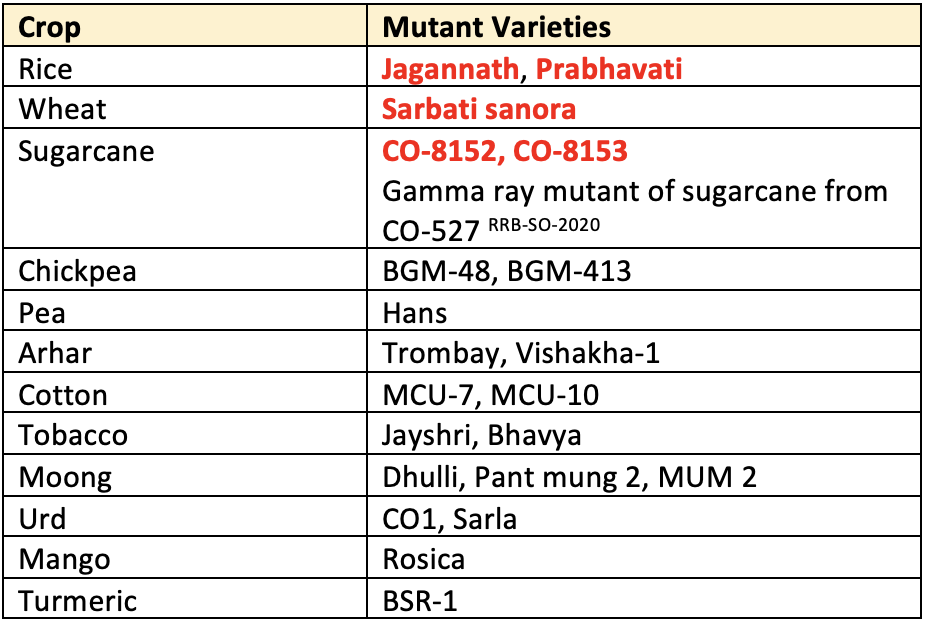
- Rice:
Polyploidy
- The somatic chromosome number of any species, whether diploid or polyploidy, is designated as 2n, and the chromosome number of gametes is denoted as n. An individual carrying the gametic chromosome number, n, is known as haploid.
- A monoploid, on the other hand, has the basic chromosome number, x. In a diploid species, n = x; one x constitutes a genome or chromosome complement. The different chromosomes of a single genome are distinct from each other in morphology and or gene content and homology; members of a single genome do not show a tendency of pairing with each other. Thus a diploid species has two, a triploid has 3 and a tetraploid has 4 genomes and so on.
- In euploids, the chromosome number is an exact multiple of the basic or genomic number. Euploidy is more commonly known as polyploidy.
- When all the genomes present in a polyploidy species are identical, it is known as autopolyploid and the situation is termed as
autopolyploidy. - In the case of allopolyploids, two or more distinct genomes are present. Euploids may have 3 (triploid), 4 (tetraploid), 5 (pentaploid), or more genomes making up their somatic chromosome number. In case of autopolyploidy, they are known as autotriploid, autotertaploid, autopentaploid, and so on, while in the case of allopolyploidy they are termed as allotriploid, allotetraploid, allopentaploid, etc.
Amphidiploidis an allopolyploid that has two copies of each genome present in it and, as a consequence, behaves as a diploid during meiosis. A segmental allopolyploid contains two or more genomes, which are identical with each other, except for some minor differences.
👉🏻 Origin and production of doubled chromosome numbers
- Spontaneous: Chromosome doubling occurs occasionally in somatic tissues and unreduced gametes are produced in low frequencies.
- Production of adventitious buds: decapitation in some plants leads to callus development at the cut ends of the stem. Such a callus has some polyploid cells and some of the shoot buds regenerated from the callus may be polyploid. In solanaceae 6-36% of adventitious buds are tetraplods. The frequency of ployploid buds may be increased by the application of 1% IAA at the cut ends as it promotes callus development.
- Treatment with physical agents: Heat or cold treatment centrifugation, X-ray or gamma ray irradiation may produce polyploid. Exposing the plants or ears of maize to a temperature of 38-45 °C at the time of the first division of zygote produce 2 -5 % tetraploid progeny.
- Regeneration in vitro: polyploidy is a common feature of the cells cultured in-vitro.
- Colchicine treatment: Colchicine treatment is the most effective and the most widely used treatment for chromosome doubling.
Autopolyploids
👉🏻 Morphological and cytological features of auto polyploids
- Polyploids have larger cell size than diploids. Guard cells of stomata are larger the number of stomata per unit area is less in polyploids than diploids.
- Pollen grains of polyploids are generally larger than those of the corresponding diploids.
- Polyploids are generally slower in growth and later in flowering.
- Polyploids usually have larger and thicker leaves, and larger flowers and fruits which are usually less in number than in diploids.
- Polyploids generally show reduced fertility due to irregularities during meiosis and due to genotypic imbalance leading to physiological disturbances.
- In many cases autopolyploidy leads to increased vigour and vegetative growth.
- Different species have different levels of optimum ploidy. For sugarbeet the optimum level is 3x, sweetpotato 6x while for timothy grass it is between 8 -10x.
- Autopolyploids generally have a lower dry matter content than diploids.
Application of Auto-polyploidy in Crop improvement Triploids
- Triploids are produced by hybridization between tetraploid and diploid strains. They are generally highly sterile, except in a few cases. This feature is useful in the production of seedless watermelons. In certain species, they may be more vigorous than the normal diploids, e.g., in sugarbeets. These two examples are described in some detail. Seedless watermelons are produced by crossing tetraploid (4x, used as female) and diploid (2x, used as male) lines, since the reciprocal cross (2x x 4x) is not successful. The triploid plants do not produce true seeds; almost all the seeds are small, white rudimentary structures like cucumber (Cucumis stivus) seeds. But few normal size seeds may occur which are generally empty. For good seed setting pollination is essential. For this purpose diploid lines are planted in the ratio 1 diploid : 5 triploid plants. There are several problem viz. genetic instability of 4x lines, irregular fruit shape, a tendency towards hollowness of fruits, production of empty seeds and the labour involved in trioploid seed production.
- Triploid sugarbeets: Among root crops triploid sugar beets apparently represent the optimum level of polyploidy because 3n plants have longer roots than diploid and also yield more sugar per unit area.
- Tetraploid rye: the advantage of tetraploid over its diploid counterpart are large kernel size, superior ability to emerge under adverse condition and higher protein content. Tetraploid rye varieties have been released for cultivation. Eg. Double steel, Tetra petkus.
❌ Limitations of autoployploidy
- Larger size autoployploiods generally contain more water and produce less dry matter content than diploids
- High sterility with poor seed setting is observed
- Due to complex segregation, progress through selection is slow
- Monoploids and triploids cannot be maintained except through clonal propagation
- The varieties cannot be produced at will
- Effects of autopolyploidy cannot be predicted
Segregation in Auto tetraploids
- Segregation in autotetraploids is much more complex than in diploids.
- Depending upon the number of dominant alleles present, they are referred as simplex (Aaaa), duplex (AAaa), triplex (AAAa), Quadruplex (AAAA) and nulliplex (aaaa).
- On selfing a simplex will produce two types of gametes Aa and aa in 1:1 ratio due to random chromosome segregation. Self pollination of such a simplex would produce. There genotypes AAaa, Aaaa and aaa in the ratio 1:2:1 giving the phenotypic ratio of 3:1.
Allopolyploidy
- Allopolyploids have genomes from
two or more speciesproduction of allopolyploids has attracted considerable attention; the aim almost always was creation of new species. Some success has been evident from the emergence of triticale. Raphano brassica and allopolyploids of forage grasses. - Morphological and extological features of allopolyploids
- Allopolyploids combine the morphological and physiological characteristics of the parent species but it is very difficult to predict the precise combination of characters that would appear in the new species.
- Many allopolyploids are apomictic Ex: Tulips, Solanum
- The chromosome pairing in the new species depends upon the similarities between the chromosomes of the parental species. Chromosomes with such similarities are known as homologous chromosomes. After chromosome doubling, the allopolyploid would have two homologous chromosomes for each chromosome present in the F1 hybrid, comparable to the diploid species. Such allopolyploid is referred as amphidiploid or Allotetra ploid.
- Fertility of Allopolyploids can be improved by hybridization and selection.
Application of allopolyploidy in crop improvement
- Utilization as a Bridging species: Amphidiploids serve as a bridge in transfer of characters from one species to a related species, generally from a wild species to cultivated species. An example of use of an amphidiploid as a bridging species in the use of synthetic N.digluta or transfer of resistance to tobacco mosaic virus from N.glutinosa to N.tabacum. The F1 hybrid from the cross N.tabacum x N.glutinosa is sterile. Chromosome doubling of the F1 hybrid produces the synthetic allehexaploid N.digluta which is reasonably fertile. N. digluta is backcrossed to the recipient species (N.tabacum) to produce a pentaploid having complete somatic chromosome complement of N.tabacum and one genome of N.glutinosa. The pentaploid is sufficiently fertile to be backcrossed to N.tabaccum in the progeny N.tabacum like plants resistant to tobacco mosaic are selected and cytologically analysed.
- Creation of New crop species
- Ex: Triticales, Raphanobrassica
- Triticum turgidum x secale cereale.
- Widening the genetic base of existing Allopolyploids: The genetic base of some natural allopolyploids may be narrow, and it may be useful to introduce variability in such cases by producing the allopolyploids afresh from their parental species. B. napus is a case in point; the genetic variability of this species is narrow and the only recourse available is to synthesize new allopolyploid B.napus to widen its genetic base. This is being done by crossing B.campestris (n=10, AA) with B.oleracea (n=9, CC), the parental diploid species, to produce the amphidiploid B.napus (n=19, AACC). The two species, B.campestris and B.olerancea, have to be crossed as autotetraploids; the cross is very difficult and embryo culture has to be used; somatic hybridization is being used to get around these problems.
❌ Limitations of Allopolyploidy
- The effects of allopolyploidy cannot be predicted. The allopolyploids have some features from both the parental species, but these features may be the undesirable ones, e.g., Raphanobrassica, or the desirable ones, e.g., Triticale.
- Newly synthesized allopolyploids have many defects, e.g., low fertility, cytogenetic and genetic instability, other undesirable features etc.
- The synthetic allopolyploids have to be improved through extensive breeding at the polyploidy level. This involves considerable time, labour and other resources.
- Only a small proportion of allopolyploids are promising; a vast majority of them are valueless for agricultural purposes.
- When mutations are induced for crop improvement, the entire operation of the induction and isolation, etc. of mutants is called mutation breeding.
- Seeds, pollen grains or vegetative propagules (buds & cuttings) may be used for mutagenesis. In sexually propagated crops, seeds are the most commonly used plant part. In case of clonal crops buds or cuttings are used for mutagenesis. Chemical mutagens are best used with seeds.
- Whole plants are generally irradiated during the flowering stage so that it is equivalent to irradiation of pollen grains and egg cells. Treatment of whole plants requires special facilities i.e. a gamma garden.
Gamma garden
- It is an area subjected to gamma-irradiation. This area is enclosed by thick high walls. The purpose of y-garden is to irradiate whole plants …
Become Successful With AgriDots
Learn the essential skills for getting a seat in the Exam with
🦄 You are a pro member!
Only use this page if purchasing a gift or enterprise account
Plan
- Unlimited access to PRO courses
- Quizzes with hand-picked meme prizes
- Invite to private Discord chat
- Free Sticker emailed
Lifetime
- All PRO-tier benefits
- Single payment, lifetime access
- 4,200 bonus xp points
- Next Level
T-shirt shipped worldwide

Yo! You just found a 20% discount using 👉 EASTEREGG

High-quality fitted cotton shirt produced by Next Level Apparel